Vibrational (Infrared) Spectroscopy - PowerPoint PPT Presentation
1 / 26
Title:
Vibrational (Infrared) Spectroscopy
Description:
Vibrational (Infrared) Spectroscopy vibrational modes C O equilibrium bond distance re can be changed by applying energy potential well for modified ... – PowerPoint PPT presentation
Number of Views:191
Avg rating:3.0/5.0
Title: Vibrational (Infrared) Spectroscopy
1
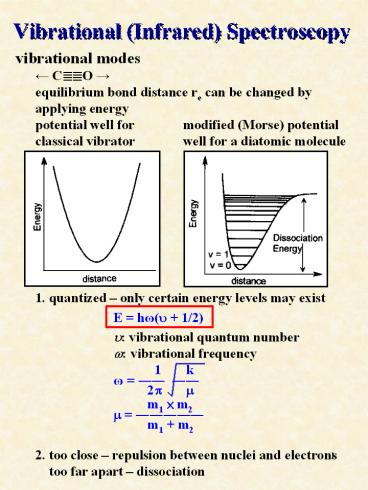
Vibrational (Infrared) Spectroscopy
- vibrational modes
- ? C??O ?
- equilibrium bond distance re can be changed by
- applying energy
- potential well for modified (Morse)
potential - classical vibrator well for a diatomic
molecule - 1. quantized only certain energy levels may
exist - E h?(u 1/2)
- u vibrational quantum number
- w vibrational frequency
2
ex. HCl u(HCl) 2990 cm-1 DCl
u(DCl) 2145 cm-1 ex. u(NO) bond
order NO 2273 cm-1 3 NO 1880
cm-1 2.5 NO- 1365 cm-1 2 NO2- 886
cm-1 1.5 number of vibrational modes a
molecule consists of N atoms, there are 3N
degrees of freedom translation
rotation vibration nonlinear 3 3
3N 6 linear 3 2
3N 5 type of vibrational modes
stretching mode u bending mode
d IR active absorption Raman active
absorption
3
- frequencies for some commonly encountered groups,
fragments, - and linkages in inorganic and organic molecules
4
- ex. W(CO)6
Mn(CO)5Br - compound u(CO) (cm-1)
- Ti(CO)62- 1740
- V(CO)6- 1860
- Cr(CO)6 2000
- Mn(CO)6 2095
- stretching modes of CO and IR frequencies
- (a) terminal (b) doubly bridging (c) triply
bridging
5
- some ligands capable of forming linkage isomers
- IR spectrum for nujol
721
1377 1462
2925 2855
6
- symmetry of normal vibrations
- ex. CO32- 6 vibrational modes
- C3(u3a) -1/2u3a 1/2 u3b
- C3(u3b) -3/2u3a - 1/2 u3b
7
- C3
- c 0
- C2
8
- G A1 A2 3E 2A2 E
- 3 translatory modes E, A2
- 3 rotational modes A2, E
- genuine vibrational modes Gg A1 2E A2
- IR active E, A2 (3 bands)
- Raman active A1, E (3 bands)
- particular internal coordinates to normal modes
- CO bonds
- E C3 C2 sh S3
sv - G 3 0 1 3 0
1 - GCO A1 E in-plane
stretching
9
- (i) trans-M(CO)4L2
- D4h E C4 C2 C2 C2 i S4 sh
sv sd - L 4 0 0 2 0 0
0 4 2 0 - OC CO
- OC CO gt A1g B1g Eu
- L IR-active Eu
- (ii) cis-M(CO)4L2
- CO C2v E C2 s s
- OC L 4 0 2 2
- OC L gt 2A1 B1 B2
- CO IR-active 2A1, B1,
B2 - (iii) mer-M(CO)3L3
- CO C2v E C2 s s
- L L 3 1 1
2 - OC L gt 2A1 B1
- OC IR-active 2A1, B1
10
- (v) M(CO)4L
- L D3v E C3 sv
- 4 1 2
- gt 2A1 E
- IR-active 2A1, E
- D2v E C3 sv sv
- L 4 0 2 2
- gt 2A1 B1 B2
- IR-active 2A1, B1, B2
- (vi) M(CO)3L2
- L D3h E C3 C2 sh S3
sv - 3 0 1 3 0
1 - gt A1 E
- L IR-active E
- L Cs E sh
- 3 1
11
- number of CO stretching bands in IR spetcrum for
metal carbonyl compounds
12
number of IR bands of some common geometric
arrangements
13
- calculation of force constants
- for diatomic molecule AB harmonic oscillator
- f m-1 l 0
- for polyatomic molecule
- Wilsons method The F and G matrix method
- FG El 0
- F matrix of force constant (potential energy)
- G matrix of masses and spatial relationship of
- atoms (kinetic energy)
- E unit matrix
- e.g. H2O Gg 2A1 B1
- 2 O-H distance Dd1, Dd2 A1 B1
- ?HOH D? A1
- using projection operator to obtain complete
set - of symmetry coordinates for vibrations
- A1 S1 D?
- S2 1/v2(Dd1 Dd2)
- B1 S3 1/v2(Dd1 - Dd2)
14
- 2V fd(Dd1)2 fd(Dd2)2 f?(D?)2 2 fdd(Dd1
Dd2) - 2 fd?(Dd1 D?) 2 fd?(Dd2 D?)
- Dd1 Dd2 D? fd fdd fd?
Dd1 - fdd fd fd? Dd2
- fd? fd? f? D?
- sf s
- relationship between the internal coordinates
and - the symmetry coordinates
- S U s
- U matrix
- Dq 0 0 1
Dd1 - Dd1 Dd2 1/v2 1/v2 0
Dd2 - Dd1 - Dd2 1/v2 -1/v2 0
Dq - S U s s U S s (U S) SU
- sfs SFS
- (SU)f(US) SFS
- S(UfU)S SFS gt F UfU
15
- G matrix G UgU
- 0 0 1 gd gdd gd?
0 1/v2 1/v2 - G 1/v2 1/v2 0 gdd gd
gd? 0 1/v2 -1/v2 - 1/v2 -1/v2 0 gd? gd? g?
1 0 0 - g33 v2 g13 0
- v2 g13 g11 g12 0
- 0 0 g11 - g12
- g11 mH mO
- g12 mO cos?
- g13 -(mO/r) sin?
- g33 2(mH mO - mO cos?)/r2
- m reciprocal of the mass
- 2(mH mO - mO cos?)/r2 -(v2mO/r)
sin? 0 - G -(v2mO/r) sin? mH mO
(1 cos?) 0 - 0
0 mH mO (1 - cos?) - for H2O ? 104.3o31 r 0.9580 Å
- 2.332 -0.0893 0
- G -0.0893 1.0390 0
16
- elements of the g matrix
- mi reciprocal mass of the ith atom
- rij reciprocal of the distance between ith
and jth
17
- Raman spectroscopy
- light of energy less than that required to
- promote a molecule into an excited electronic
- state is absorbed by a molecule,
- a virtual excited state is created
- virtual state is very short lifetime, the
majority - of the light is re-emitted over 360oC, this is
- called Rayleigh scattering
- C. V. Raman found that the energy of a small
- proportion of re-emitted light differs from the
- incident radiation by energy gaps that
- correspond to some of the vibrational modes
- Stokes lines
- anti-Stokes line
18
- schematic representation of Raman spectrometer
- selection rules for vibrational transitions
- a fundamental will be infrared active if the
- normal mode which is excited belongs to the
- same representation as any one or several of
- the Cartesian coordinates
- a fundamental will be Raman active if the
- normal mode involved belongs to the same
- representation as one or more of the
- components of the polarizability tensor of the
- molecule
- the exclusion rule in centrosymmetric
molecules,
19
- ex. Na2MoO4 dissolved in HCl exhibits Raman
- peaks at 964, 925, 392, 311, 246, 219 cm-1
- 925, 311 cm-1 being polarized
- what can be deduced from the spectrum?
- no n(MoH) and n(OH) bands
- only MCl and MO likely exist
- 964, 925 cm-1 MoO stretching bands
- 392 cm-1 MoO bending mode
- 311, 246, 219 cm-1
- MoCl
stretching modes - possible product
20
- normal vibrational modes for common structures
21
(No Transcript)
22
(No Transcript)
23
(No Transcript)
24
(No Transcript)
25
(No Transcript)
26
(No Transcript)