Lecture 27. Debye Model of Solids, Phonon Gas - PowerPoint PPT Presentation
Title:
Lecture 27. Debye Model of Solids, Phonon Gas
Description:
Lecture 27. Debye Model of Solids, Phonon Gas In 1907, Einstein developed the first quantum-mechanical model of solids that was able to qualitatively describe the low ... – PowerPoint PPT presentation
Number of Views:270
Avg rating:3.0/5.0
Title: Lecture 27. Debye Model of Solids, Phonon Gas
1
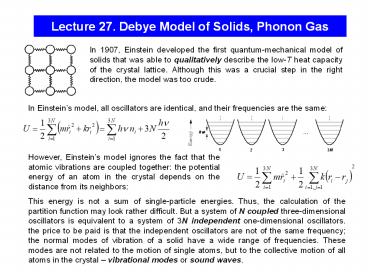
Lecture 27. Debye Model of Solids, Phonon Gas
In 1907, Einstein developed the first
quantum-mechanical model of solids that was able
to qualitatively describe the low-T heat capacity
of the crystal lattice. Although this was a
crucial step in the right direction, the model
was too crude.
In Einsteins model, all oscillators are
identical, and their frequencies are the same
However, Einsteins model ignores the fact that
the atomic vibrations are coupled together the
potential energy of an atom in the crystal
depends on the distance from its neighbors
This energy is not a sum of single-particle
energies. Thus, the calculation of the partition
function may look rather difficult. But a system
of N coupled three-dimensional oscillators is
equivalent to a system of 3N independent
one-dimensional oscillators. the price to be
paid is that the independent oscillators are not
of the same frequency the normal modes of
vibration of a solid have a wide range of
frequencies. These modes are not related to the
motion of single atoms, but to the collective
motion of all atoms in the crystal vibrational
modes or sound waves.
2
Einsteins Model of a Solid
In 1907 Einstein, in the first application of
quantum theory to a problem other than radiation,
modeled a solid body containing N atoms as a
collection of 3N harmonic oscillators. The
partition function of a single oscillator
The oscillators are independent of each other,
thus
The mean energy
This looks familiar the same energy would have a
photon of frequency ?.
The internal energy is not a directly measurable
quantity, and instead we measure the heat
capacity
equipartition
high T (kBTgtgth?)
Limits
low T (kBTltlth?)
The Einstein model predicts much too low a heat
capacity at low temperatures!
3
Debyes Theory of the Heat Capacity of Solids
Debyes model (1912) starts from the opposite
point of view, treating the solid as a continuum,
i.e., the atomic structure is ignored. A
continuum has vibrational modes of arbitrary low
frequencies, and at sufficiently low T only these
low frequency modes are excited. These low
frequency normal modes are simply standing sound
waves.
Nobel 1936
If we quantize this elastic distortion field,
similar to the quantization of the e.-m. field,
we arrive at the concept of phonons, the quanta
of this elastic field. For the thermal phonons,
the wavelength increases with decreasing T
cS the sound velocity
These low-energy modes remain active at low
temperatures when the high-frequency modes are
already frozen out. Large values of ? that
correspond to these modes justify the use of a
continuum model.
There is a close analogy between photons and
phonons both are unconserved bosons.
Distinctions (a) the speed of propagation of
phonons ( the speed of sound waves) is by a
factor of 105 less than that for light, (b) sound
waves can be longitudinal as well as transversal,
thus 3 polarizations (2 for photons), and (c)
because of discreteness of matter, there is an
upper limit on the wavelength of phonons the
interatomic distance.
4
Density of States in Debye Model
For a macroscopic crystal, the spectrum of sound
waves is almost continuous, and we can treat ? is
a continuous variable. As in the case of photons,
we start with the density of states per unit
frequency g(?). The number of modes per unit
volume with the wave number lt k
- multiplied by 3 since a sound wave in a solid
can have three polarizations (two transverse and
one longitudinal).
- this eq. only holds for sufficiently low ?
( large wavelengths and the continuous
approximation is valid). There is also an upper
cut-off for the frequencies (? ? interatomic
distances), the so-called Debye frequency ?D,
which depends on the density n
cS 3 km/s, a0.2 nm, ?D1013 Hz
Each normal mode is a quantized harmonic
oscillator. The mean energy of each mode
and
is the total energy per unit volume. The U0 term
comes from the zero-point motion of atoms. It
reduces the cohesive energy of the solid (the
zero point motion in helium is sufficient to
prevent solidification at any T at normal
pressure), but since it does not depend on T, it
does not contribute to C. Note that we ignored
this term for phonons, where it is ?. In QED,
this unobservable term is swept under the rug by
the process known as renormalization.
5
The Heat Capacity in Debyes Model
At low temperatures, we can choose the upper
limit as ? (the high-frequency modes are not
excited, the energy is too low). How low should
be T
The low-T heat capacity
Thus, Debyes model predicts that in the limit of
sufficiently low T, the heat capacity due to
vibrations of the crystal lattice (in a metal
electrons also contribute to C) must vary as T3,
and not as ?2exp(- h?/kBT), as in Einsteins
model. (Roughly speaking, the number of phonons
T3, their average energy is proportional to T).
At high temperatures, all the modes are excited
(the number of phonons does not increase any
more), and the heat capacity approaches the
equipartition limit, C3NkB .
6
Debye Temperature
The material-specific parameter is the sound
speed. If the temperature is properly normalized,
the data for different materials collapse onto a
universal dependence
The normalization factor is called the Debye
temperature
It is related to the maximum frequency ?D, the
Debye frequency
The higher the sound speed and the density of
ions, the higher the Debye temperature. However,
the real phonon spectra are very complicated, and
?D is better to treat as an experimental fitting
parameter.
7
Problem (blackbody radiation)
The spectrum of Sun, plotted as a function of
energy, peaks at a photon energy of 1.4 eV. The
spectrum for Sirius A, plotted as a function of
energy, peaks at a photon energy of 2.4 eV. The
luminosity of Sirius A (the total power emitted
by its surface) is by a factor of 24 greater than
the luminosity of the Sun. How does the radius of
Sirius A compare to the Suns radius?
The temperature, according to Wiens law, is
proportional to the energy that corresponds to
the peak of the photon distribution.
8
Final 2006 (blackbody radiation)
The frequency peak in the spectral
density of radiation for a certain distant star
is at 1.7 x 1014 Hz. The star is at a distance of
1.9 x 1017 m away from the Earth and the energy
flux of its radiation as measured on Earth is
3.5x10-5 W/m2. a) (5) What is the surface
temperature of the star? b) (5) What is the
total power emitted by 1 m2 of the surface of the
star? c) (5) What is the total electromagnetic
power emitted by the star? d) (5) What is the
radius of the star?
(a)
(b)
(c)
(d)
9
Problem 2006 (blackbody radiation)
- The cosmic microwave background radiation (CMBR)
has a temperature of approximately 2.7 K. - (a) (5) What wavelength ?max (in m) corresponds
to the maximum spectral density u(?,T) of the
cosmic background radiation? - (5) What frequency ?max (in Hz) corresponds to
the maximum spectral density u(?,T) of the cosmic
background radiation? - (5) Do the maxima u(?,T) and u(?,T) correspond
to the same photon energy? If not, why? - (d) (15) What is approximately the number of
CMBR photons hitting the earth per second per
square meter i.e. photons/(s?m2)?
(a)
(b)
(c)
The maxima u(?,T) and u(?,T) do not correspond to
the same photon energy. As ? increases, the
frequency range included in unit wavelength
interval increases as ?2, moving the peak to
shorter wavelengths
10
Problem 2006 (blackbody radiation) cont.
(d)
11
Problem (blackbody radiation)
The planet Jupiter is a distance of 7.78 x 1011
meters from the Sun and is of radius 7.15 x 107
meters. Assume that Jupiter is a blackbody even
though this is not entirely correct. Recall that
the solar power output of Sun is 4 x 1026 W.
a) What is the energy flux of the Sun's
radiation at the distance of Jupiter's orbit?
b) Recognizing that Sun's energy falls on the
geometric disk presented to the Sun by the
planet, what is the total power incident on
Jupiter from the Sun?
c) Jupiter rotates at a rather rapid rate (one
revolution per 0.4 earth days) and therefore all
portions of the planet absorb energy from the
Sun. Hence all portions of the surface of this
planet radiate energy outward. On the basis of
this information find the surface temperature of
Jupiter.
12
d) Estimates of the surface temperature of
Jupiter indicate that it is clearly above 500
Kelvins. On the basis of your answer to c) what
might you conclude about this planet? There
must be some other source of energy to produce a
surface temperature of 500 K.
13
Problem (blackbody radiation)
Tungsten has an emissivity of 0.3 at high
temperatures. Tungsten filaments operate at a
temperature of 4800 K. a) At what frequency
does a tungsten filament radiate the most energy?
b) What is the power/unit surface emitted by
the tungsten filament? c) If the surface area
of a tungsten filament is 0.01 cm2 what is the
power output of the bulb? d) What is the
energy flux of radiation emitted by the filament
2 meters from the bulb? e) What fraction of
radiation incident on a tungsten filament is
reflected? Answers a) 2.88 x 1014 Hz, b) 9.03
x106 W/m2, c) 9.03 watts, d) 0.180 watts, and e)
70.
14
Problem (radiation pressure)
- At what temperature will the pressure of the
photon gas be equal to 105 Pa ( one bar )? - At what temperature will the pressure of the
photon gas be equal to 10-5 Pa? - The temperature at the Suns center is 107 K.
What is the pressure of the radiation? Compare it
to the pressure of the gas at the center of the
Sun, which is 1011 bar. - Calculate the pressure of the Suns radiation on
the Earths surface, given that the power of the
radiation reaching earth from the Sun is 1400
W/m2.
(a)
P105 Pa
P10-5 Pa
(b)
(c)
- at this T, the pressure of radiation is still
negligible in the balance for mechanical
equilibrium
(d)
15
Problem (blackbody radiation)
The black body temperature is 3000K. Find the
power emitted by this black body within the
wavelength interval ?? 1 nm near the maximum of
the spectrum of blackbody radiation.
the power emitted by a unit area in all
directions
near the maximum