PVC and Plasticizers - PowerPoint PPT Presentation
1 / 16
Title:
PVC and Plasticizers
Description:
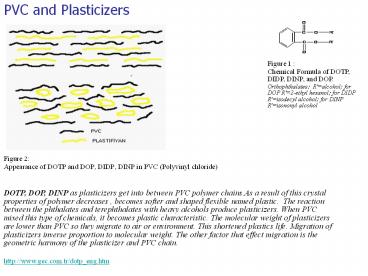
Figure 1 : Chemical Formula of DOTP, DIDP, DINP, and DOP. Orthophthalates: R'=alcohol; for DOP R'=2-ethyl hexanol; for DIDP R'=isodecyl alcohol; for DINP R'=isononyl ... – PowerPoint PPT presentation
Number of Views:1112
Avg rating:3.0/5.0
Title: PVC and Plasticizers
1
PVC and Plasticizers
Figure 1 Chemical Formula of DOTP, DIDP, DINP,
and DOP. Orthophthalates R'alcohol for DOP
R'2-ethyl hexanol for DIDP R'isodecyl alcohol
for DINP R'isononyl alcohol
- Figure 2
- Appearance of DOTP and DOP, DIDP, DINP in PVC
(Polyvinyl chloride)
DOTP, DOP, DINP as plasticizers get into between
PVC polymer chains.As a result of this crystal
properties of polymer decreases , becomes softer
and shaped flexible named plastic. The reaction
between the phthalates and terephthalates with
heavy alcohols produce plasticizers. When PVC
mixed this type of chemicals, it becomes plastic
characteristic. The molecular weight of
plasticizers are lower than PVC so they migrate
to air or environment. This shortened plastics
life. Migration of plasticizers inverse
proportion to molecular weight. The other factor
that effect migration is the geometric harmony of
the plasticizer and PVC chain. http//www.gec.com
.tr/dotp_eng.htm
2
PVC Plastic
With 3-4 million tones of DEHP being produced
annually, the phthalate is causing increasing
alarm as a pollutant in many countries. 95 of
DEHP produced is used as PVC plasticizers. The
general population is exposed to DEHP since PVC
is used in such diverse application and that DEHP
is leaches through consumer products. DEHP, bound
by only weak van der Waals forces, is not
chemically bound to PVC and so is prone to
leaching into the environment. DEHP emission
occurs during plastic production, use, and after
disposal. Once disposed of, DEHP does not undergo
significant physio-chemical degradation. In
medical applications, DEHP can leach out from the
materials and travel into the media. There has
been increasing concern regarding how DEHP
exposure impacts human health through medical
procedures such as intravenous therapy, enteral
and parenteral nutrition support, blood
transfusion, hemodialysis, and extracorporeal
membrane oxygenation, and how exposure impacts
the environment and wildlife through leaching
from water pipes and landfills. Though DEHP is
suggested to be of low acute toxicity, long-term
exposure may have an adverse effect on human
health. Studies of risk assessment have shown
that DEHP has critical effects on fertility,
endocrine system, kidneys and development. The
Food and Drug Administration has issued a report
acknowledging that PVC medical devices are a
concern to critically ill infants. Furthermore,
studies suggest that chemicals with the ability
to disrupt the endocrine system are a potential
threat to the health of humans, aquatic animals,
and wildlife. For aquatic organisms, there have
been several cases of documentation on the
adverse effects of DEHP on Daphnia and fish
species. Another DEHP study has pointed towards
the perturbation of normal metabolism in liver,
heart, testes, adrenal gland, and brain in
mammals such as rats, rabbits, and pigs. The
adverse effects of DEHP pose potential
environmental and health risks through leaching,
making DEHP harmful in terms of production, use,
and disposal. To address the source of the
problem, we hoped to reduce emissions of DEHP by
finding suitable, safe, and biodegradable
alternative PVC plasticizers. PVC
Bioplasticizers by Robyn Thom and Thomas
Sunhttp//www.odec.ca/projects/2007/sunt7t2/purpos
e.htm
Wypych, George, Handbook of Plasticizers,
2004. Figure 1 Polymerization reaction of PVC.
Figure 2 Diagram showing plasticization.
3
PVC and Plasticizers
Chemical Formula of DOTP, DIDP, DINP, and
DOP. Orthophthalates R'alcohol for DOP
R'2-ethyl hexanol for DIDP R'isodecyl alcohol
for DINP R'isononyl alcohol
4
General Vulcanization
- (a) Unvulcanized natural rubber molecules have
few if any cross-links. - (b) Vulcanized rubber has a network structure
with cross-links - (c) Vulcanized rubber on stretching.
We can form covalent bonds between the polymer
molecules, and if we do this the material will
become much more rigid because the chains are no
longer free to move apart. The more cross-links
between chains, the more rigid the rubber until
eventually the polymer is so cross-linked that it
is no longer rubbery because there is no
flexibility of the chains between the
cross-links. Goodyears vulcanization process
produces a controlled amount of crosslinking. The
sulfur reacts with the double bonds and forms
sulfur bridges as cross-links between the chains,
resulting in a huge three-dimensional network as
you can see here. BBC/OU Open2.net - The World
Around Us - Lost at sea - Explore the science -
Rubber ...
5
True Reel-to-reel Plasma Treatment Under Ambient
Conditions.
Tony Herbert of Dow Corning ( Ireland ) explained
plasma treatment as developed by Plasma Solutions
now part of DC. By using vertical electrodes
the operation can be carried out in a helium
atmosphere with minimal helium loss and avoidance
of oxidative reactions that limit the usefulness
of corona treatments. A wide range of polymer
precursors can be injected directly into the
helium glow discharge to get soft polymerisation
onto the fabric. (see Patent WO
02/28548). Alkenes and acrylates polymerise by
free-radical mechanisms while cyclic siloxanes
undergo ring-opening polymerisation. The latter
allow hydrophobic surfaces with slippery soft
textures to be coated onto otherwise hydrophilic
materials. Octa-methyl cyclo tetra siloxane has
been used under helium to give cotton total
hydrophobicity. However in the presence of
oxygen, the plasma breaks it down into silicates
which increase hydrophilicity. Also for
hydrophilicity, acrylic acid has been polymerised
onto polypropylene to give it an absorbent and
reactive surface. Heptadecafluorodecene and other
fluoro compounds have been polymerised onto
fabrics to confer oleophobicity. Other polymer
precursors mentioned were Colloidal metals
for optical or conductivity enhancement
Thiophene or Pyrrole for conductivity Mixed
monomers for copolymer coatings and pH
control. The system allows high deposition rates
and hence high productivity in a true
reel-to-reel treatment under ambient
conditions. http//www.nonwoven.co.uk/reports/TT2
0200320contents.html
6
Sulfur Cross-Linking Goodyear Vulcanization
Process
Vulcanization methods A variety of methods exist
for vulcanization. The economically most
important method (the vulcanization of tires)
uses high pressure and temperature. A typical
vulcanization temperature for a passenger tire is
10 minutes at 170 C. This type of vulcanization
is called compression molding. The rubber article
is intended to adopt the shape of the mold. Other
methods, for instance to make door profiles for
cars, use hot air vulcanization or microwave
heated vulcanization (both continuous
processes). Four types of curing systems are in
common use. They are 1. Sulfur systems 2.
Peroxides 3. Urethane crosslinkers 4. Metallic
oxides
The reactive sites - "cure sites" - are allylic
hydrogen atoms. These CH bonds are adjacent to
carbon-carbon double bonds. During vulcanization,
some of these C-H bonds are replaced by chains of
sulfur atoms that link with a cure site of
another polymer chain. These bridges contain
between one and eight atoms. The number of sulfur
atoms in the crosslink strongly influences the
physical properties of the final rubber article.
Short crosslinks give the rubber better heat
resistance. Crosslinks with higher number of
sulfur atoms give the rubber good dynamic
properties but with lesser heat resistance.
Dynamic properties are important for flexing
movements of the rubber article, e.g., the
movement of a side-wall of a running tire.
Without good flexing properties these movements
will rapidly lead to formation of cracks and,
ultimately, to failure of the rubber article.
7
Cross-Linking or Curing
- Four types of curing systems are in common use.
- They are
- Sulfur systems
- Peroxides Peroxide curing (HTV rubber)
- To carry out peroxide curing, it is first
necessary to generate free radicals. This can be
done either with heat or with radiation. - Different organic peroxides may serve as
free-radical generators for initiating this type
of curing. - Urethane crosslinkers
8
Properties Changed by Vulcanization
The reactive sites - "cure sites" - are allylic
hydrogen atoms. These CH bonds are adjacent to
carbon-carbon double bonds. During
vulcanization, some of these C-H bonds are
replaced by chains of sulfur atoms that link with
a cure site of another polymer chain. These
bridges contain between one and eight atoms. The
number of sulfur atoms in the crosslink strongly
influences the physical properties of the final
rubber article. Short crosslinks give the rubber
better heat resistance. Crosslinks with higher
number of sulfur atoms give the rubber good
dynamic properties but with lesser heat
resistance. Dynamic properties are important for
flexing movements of the rubber article, e.g.,
the movement of a side-wall of a running tire.
Without good flexing properties these movements
will rapidly lead to formation of cracks and,
ultimately, to failure of the rubber article.
9
PVC Properties
10
Plasma Polymers
- Film structure
- In most cases plasma polymers are highly
cross-linked and have a disordered structure.
Structural preservation and cross-linking
gradients can be controlled through process
parameters, such as pressure, working gas-flow
and applied electrical output so one can also
construct so-called gradient layers i.e with
increasing degree of cross-linking over
thickness. - Special layer characteristics that are qualified
for a multitude of applications - excellent coating adhesion on almost all
substrates - chemical, mechanical and thermal stability
- high barrier effect
- Applications from plasma polymer coatings are as
follows - scratch resistant coatings (displays or windows)
- corrosion protection
- barrier layers
- GK Polymer thin films -20- Vorlesung/Lecture
Hans-Ulrich Krebs
Water drop on a surface being coated by
plasma-polymerization
11
- Crack-resistant anticorrosive coatings based on
vulcanized water dispersion of chlorine-sulphopoly
ethylene - Biaxially oriented PET film can be metallized by
vapor deposition of a thin film of evaporated
aluminum, gold, or other metal onto it. - The result is much less permeable to gasses
(important in food packaging) and reflects up to
99 of light, including much of the infrared
spectrum. For some applications like food
packaging, the aluminized boPET film can be
laminated with a layer of polyethylene, which
provides sealability and improves puncture
resistance. The polyethylene side of such a
laminate appears dull and the PET side shiny. - Other coatings, such as conductive indium tin
oxide (ITO), can be applied to boPET film by
sputter deposition. - Metallized nylon (or "foil") balloons used for
floral arrangements and parties are often
mistakenly called "Mylar", one of the trade names
for boPET film. - Studies of Wettability of Medical PVC by Remote
Nitrogen Plasma - Changes morphology and composition incorporation
of N and contact angle from 89 to 18 deg - Atmospheric RF plasma effects on the film
adhesion property - Polymers in thin film form were used for
modification by atmospheric RF plasma influence
of the plasma treatments using Ar and ArO2 on
surface energy, morphology, chemistry structure
of the films was investigated. - The reactions of Ti with the polymer formed of
TiCl2, TiC, Ti-oxide contributed to film
adhesion. - In comparison with Ar, the mixed ArO2 RF plasma
treatment was a more timesaving process and had
more influences on surface activation and film
adhesion. - Oxygen plasma-vulcanized deformable PDMD sheet
culture substrates - A method of preparing deformable
polydimethylsiloxane sheet culture substrates by
oxygen plasma vulcanization was developed.
12
- Adhesive Bonding Energizing Low-Surface Energy
Plastics - Polypropylene is used in a wide range of
assemblies, because of its toughness,
flexibility, resistance to heat, moisture,
chemicals - However, properties that make polypropylene good
for containing hot water and battery acid also
make it difficult to bond with adhesives. - Plastic has such low surface energyjust 30 dynes
per square centimeter, depending on the
formulationthat adhesives cant adequately wet
the surface to get a grip, whether chemical or
mechanical. - To bond polypropylene and other resistant
plastics, engineers need to raise the materials
surface energy. One way to do that is by exposing
the surface of the plastic to plasma, the
so-called fourth state of matter. - Plasma polymerization of sulfur to decrease the
blooming effect and its effect on vulcanization
with different accelerators. - SURFACE MODIFICATIONS AND ADHESION OF VULCANIZED
SBR RUBBER TREATED WITH RF PLASMAS OF DIFFERENT
GASES - The surface modifications produced by treatment
of a synthetic vulcanized styrenebutadiene rubber
(R1) with oxidizing (oxygen, air, carbon dioxide)
and nonoxidizing (nitrogen, argon) RF plasmas
have been assessed by ATR-IR and XPS
spectroscopy, SEM, and contact angle
measurements. - The effectiveness of the treatment depended on
the gas atmosphere used to generate the RF
plasma. In general, acceptable adhesion values of
treated R1 rubber were obtained for all plasmas,
except for the nitrogen plasma treatment during
15 min, due to the creation of weak layers of low
molecular weight moieties on the outermost R1
rubber layer. - On the other hand, the air, carbon dioxide, and
oxygen plasmas produced ablation of the R1 rubber
surface, whereas mechanical degradation was not
produced by treatment with the Ar plasma. - Plasma Surface Treatment Oxygen Plasma
Treatment - Surface tension is invisible to the naked eye,
but it can seriously impact an objects function.
Low surface energy or contaminants can prevent
the bonding of inks and adhesives, limiting the
materials range of use. Thats why industries in
plastics, metal and other fields use plasma
treatments for etching, cleaning and activating
surfaces. - Atmospheric plasma surface treatments are
available in a variety of configurations Oxygen
plasma treatment Flame plasma treatment and
Variable chemical plasma treatment
13
Polymer thin films Vorlesung/Lecture Hans-Ulrich
Krebs
- Microscopical View
- at the surface neighbors are missing (in
comparison to bulk) -change of the structure near
the surface - chain conformation near the surface Orientation
of chain segments parallel to the surface - distribution of chain ends Enrichment of chain
ends at the surface due to entropic effects - distribution of the segments strong orientation
by breaking the translation invariance - influence on kinetics mobility at the surface
parallel to the surface is strongly increased
within a thickness of about 2 nm -reason less
entanglement
14
Polymer thin films Vorlesung/Lecture Hans-Ulrich
Krebs 1
- A) Formation of polymer films from solution
- we go from very simple techniques for thick films
to more and more sophisticated techniques for
thin films, even to multilayers ("it is an art,
design to prepare them") and single monolayers. - Solvent casting, painting
- most simple technique, preparation of relatively
thick films gtmm polymer solution is deposited
on a substrate drying solid film for
instance one takes a brush and simply paints the
solution on a substrate (wall) is of large
technical importance coatings of houses, - Thermal spray processing of polymers ("gun")
- Thermal spraying of polymers is gaining increased
attention because the ability to apply coatings
of polymers onto a wide variety of materials is
seen as an effective method to produce protective
barrier coatings. - Polymers that have been sprayed to date include
PE, PMMA, EMMA, PEEK, PPS, LCP, nylon, phenolic,
epoxy, Tefzel, and post consumer commingled
polymer. - Polymer powder is injected into a heat source
(flame or plasma) and transported to a preheated
substrate. - Spin coating
- Polymer is dissolved in a volutile solvent and
the solution is spinned Spin coating is the
preferred method for application of thin, uniform
films to flat substrates. An excess amount of
polymer solution is dropped on top of a
substrate. The substrate is then rotated at high
speed at an angular velocity, w, in order to
spread the fluid by centrifugal force, reducing
fluid thickness. Rotation is continued for - some time, with fluid being spun off the edges of
the substrate, until the desired film thickness
is achieved. The solvent is usually volatile,
providing for its simultaneous evaporation. - A) Special Free Standing Polymer films
- B) Multilayers diblock copolymer thin films -
self organization - Polymers produced by surface absorption of
monolayers (SAM) - Floating Technique
- Langmuir-Blodgett films
15
Polymer thin films Vorlesung/Lecture Hans-Ulrich
Krebs 2
- B) Thin film deposition techniques
- Evaporation
- During evaporation the material is heated in UHV
in a crucible to sufficient high - temperatures. The evaporating molecules leave the
surface and condense as a thin film on a - substrate (and also on the surrounding walls).
- Sputtering
- Principle When ions/atoms hit a target with high
enough energy (for instance Ar, M40, kinetic
energy E1keV), transfer of energy and impulse
towards the target atoms takes place. Ions are
implanted into the material and partially
resputtered. - Surface particles leave the surface, as soon as
the binding energy of the target material is
overcome (sputtering). - Pused laser deposition (PLD)
- UV-excimer laser, pulsed z.B. KrF 248 nm, 30 ns,
UHV oder pressures lt1mbar, energy source is
outside the chamber - Properties of the technique stoichiometry
transfer, high deposition rate, most materials
can be prepared (in UHV or in gas environment) - Plasma Polymerization
- When introducing molecular gases into a plasma,
chemically active species are formed such as
molecules in excited states, radicals and ions.
These species can react with each other, neutral
molecules or with the surface of a substrate.
This results in deposition of a thin film. Films
resulting from organic precursors are generally
known as plasma polymers. The reaction chain
leading to a plasma polymer film is not
comparable to common polymerization reactions.
For this reason, the properties of plasma polymer
films can significantly differ from their classic
chemical counterparts.
16
Plasma Enhanced Chemical Vapor Deposition of Ti
and TiO2
PE-CVD of TiO2 A low pressure radio frequency
discharge (200Wfor 5 min) was operated in oxygen,
nitrogen/ oxygen and argon/oxygen with small
admixtures of titanium(IV)isopropoxide Titanium
isopropoxide is a chemical compound with the
formula TiOCH(CH3)24. This alkoxide of
titanium(IV) is used in organic synthesis and
materials science. Titanium isopropoxide reacts
with water to deposit titanium dioxide TiOCH(CH3
)24 2 H2O ? TiO2 4 (CH3)2CHOH This reaction
is employed in the sol-gel synthesis of
TiO2-based materials. PE-CVD of Ti A new
process for depositing titanium metal layers via
chemical vapor deposition is disclosed. The
reaction gases for the improved process are
titanium tetrachloride and a hydrocarbon gas,
which for a preferred embodiment of the process
is methane. The reaction is carried out in a
plasma environment created by a radio frequency
source greater than 10 KHz. In a preferred
embodiment of the process, highly reactive methyl
radicals (CH3 --) are formed from methane gas.
These radicals attack the titanium-chlorine
bonds of the tetrachloride molecule and form
chloromethane, which is evacuated from the
chamber as it is formed. Titanium metal deposits
on a wafer or other substrate that has been
heated to a temperature within a preferred range
of 200-500 C.