Biogeochemical Cycles and Energy Flow - PowerPoint PPT Presentation
1 / 18
Title:
Biogeochemical Cycles and Energy Flow
Description:
... forms H2SO4 in water and falls with rain to earth. and becomes available (too much causes acid rain) ... detritivores (mites, protozoans, nematodes, earthworms, ... – PowerPoint PPT presentation
Number of Views:170
Avg rating:3.0/5.0
Title: Biogeochemical Cycles and Energy Flow
1
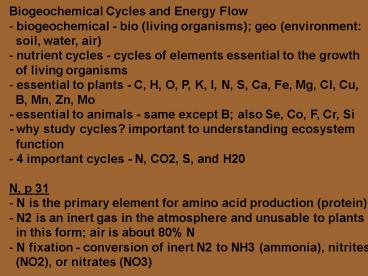
- Biogeochemical Cycles and Energy Flow
- biogeochemical - bio (living organisms) geo
(environment - soil, water, air)
- nutrient cycles - cycles of elements essential
to the growth - of living organisms
- essential to plants - C, H, O, P, K, I, N, S,
Ca, Fe, Mg, Cl, Cu, - B, Mn, Zn, Mo
- - essential to animals - same except B also Se,
Co, F, Cr, Si - - why study cycles? important to understanding
ecosystem - function
- 4 important cycles - N, CO2, S, and H20
- N, p 31
- N is the primary element for amino acid
production (protein) - N2 is an inert gas in the atmosphere and
unusable to plants - in this form air is about 80 N
- N fixation - conversion of inert N2 to NH3
(ammonia), nitrites - (NO2), or nitrates (NO3)
2
- some plants (legumes Family Fabaceae includes
beans, - peas, peanuts) and symbiotic (interacting
species that are - closely or completely dependent on each other)
bacteria in - root nodules (bacteria clusters) fix N to NH3
making it free to - plants and animals
- other free-living aerobic bacteria in soil and
blue-green algae - in aquatic systems serve the same purpose
- bacteria make NH3 by splitting N2 and combing it
with H - (very high biological energetic costs)
- non-biological fixation - lightning (chemical
reaction) and - volcanoes (by emission) produce NO3
- other changes to N - bacteria convert NH3 to NO2
and NO2 - to NO3 denitrifying bacteria in anaerobic
conditions convert - above molecules to N2 that goes back to the
atmosphere
3
(No Transcript)
4
- amount of daily fixation and release of N is
roughly equal - C, p 32
- all life on earth is carbon based all living
tissue contains C - C cycle is important for possible role in global
warming - CO2 required by plants for photosynthesis very
small - amount in atmosphere (0.03) but very important
- CO2 is produced by plants and animals thru
cellular - respiration (cell activities)
- dynamic equilibrium among C forms in water
(bicarbonates, - carbonates), sediments and fossil fuels (oil,
natural gas, - coal), atmosphere, plants, and soils
5
(No Transcript)
6
(No Transcript)
7
(No Transcript)
8
- S, p 34
- - important for forming some amino acids and
enzymes - in organic (dead plants and animals) and
inorganic (sulfur - containing rocks) deposits
- released into the environment by decomposition
of organic - matter, erosion, volcanoes, salt spray from
oceans - occurs as H2S then SO2 in air and water, and SO4
in water - and soil forms H2SO4 in water and falls with
rain to earth - and becomes available (too much causes acid
rain) - decomposers (bacteria and fungi) release sulfur
from - organic material
- plants mainly get S from SO4 in soil and water
animals by - eating plants and other animals
9
- mining (especially coal) and industry release S
and acidifies - the environment from runoff from strip mines and
burning - poor grades of coal
- H20 p 35
- hydrologic cycle - water comes from oceans to
continents - via rainfall it then evaporates, gets stored
(surface or - ground), or goes back via rivers
- not much in atmosphere and the turnover rate is
high - human activities can affect cycle reduce
aquifers or change - evaporation rates and runoff by construction of
cities
10
(No Transcript)
11
(No Transcript)
12
- Energy Flow - from sun to plants (photosynthesis)
to system - (consumers in the food web) then lost as heat
- - laws of thermodynamics
- 1) energy cannot be created or destroyed
- 2) when energy is transferred, it is transformed,
and much is - lost to unusable forms (heat)
- plants capture energy in chemical bonds which
animals in - the food web eat and pass on energy does not
cycle but - flows out of the system as heat
- - photosynthesis
- uses CO2 and H2O in a reaction by light energy
- green chlorophyll captures light energy
- sugars and O2 are produced also other organic
products - (fats, vitamins, and proteins)
- 6 CO2 6 H2O w/sunlight yields C6H12O6 6 O2
13
- decomposition - break down of organic
compounds, releases CO2 and H2O, returns
nutrients to inorganic state (vitamins and
minerals are freed from C based molecules)
done by microflora (fungi for plant litter,
bacteria for animal matter actually do the
decomposition) and detritivores (invertebrates
that feed on detritus, dead organic matter, and
really just break it down into smaller bits)
other important factors activities and ingestion
by larger organisms and leaching by
precipitation detritivores (mites, protozoans,
nematodes, earthworms, millipedes) fragment
organic material and inoculate it with bacteria
and fungi microbivores (larval beetles, flies,
mites, amoebas) feed on nutrients and energy of
microflora
14
- decomposers temporarily remove nutrients from
- circulation (nutrient immobilization)
- Food Webs and Energy Pyramids
- autotrophs - producers produce own food living
base of - food web
- heterotrophs - consumers use producers or other
- consumers as food
- trophic levels - feeding levels through which
energy is - passed begins with producers
- food chain - path of energy flow from producer
to consumer - better seen as food web
- order
- primary producers (phytoplankton and large
plants)
15
- 2) primary consumers - herbivores or prey land
and water - grazers (carp, ducks, deer, mice) and
zooplankton - 3) secondary consumer (carnivores or predators)
can go to - more levels
- omnivores - eat both autotrophs (plants) and
heterotrophs - other contributors decomposers, detritivores,
- microbivores, scavengers, saprophytes (fungi
that feed on - dead plant material decomposers), and parasites
- intricate web of energy exchange many
organisms shift - roles and can be predators and prey
- about 90 loss of energy at each step of the web
(energy is - lost as heat when chemical bonds are broken and
used to - warm bodies) 2nd law of thermodynamics
16
Relative Number Of Organisms As You Follow A Food
Web
Relative Biomass Of Organisms As You Follow A
Food Web
17
(No Transcript)
18
limits how much energy, how many individuals,
and how much biomass can accumulate up chains
called ecological pyramids fisheries managers
deal with 2nd and 3rd level consumers wildlife
managers mostly deal with 1st level consumers
(predators at higher levels cannot reach numbers
of 1st level consumers)