TOPIC IX TROPOSPHERIC AEROSOLS, ACID RAIN, - PowerPoint PPT Presentation
1 / 25
Title:
TOPIC IX TROPOSPHERIC AEROSOLS, ACID RAIN,
Description:
ACID RAIN. In addition, acid generation can occur by reactions involving dissolved O3, H2O2, ... MEASUREMENTS IN RAIN. Aerosols are solid or liquid particles ... – PowerPoint PPT presentation
Number of Views:174
Avg rating:3.0/5.0
Title: TOPIC IX TROPOSPHERIC AEROSOLS, ACID RAIN,
1
TOPIC IXTROPOSPHERIC AEROSOLS, ACID RAIN,
VISIBILITY
2
ACID RAIN
Acid rain Rain that is more acidic than
normal Acid precipitation Wet (i.e.deposition
associated with precipitation) Dry (I.e. uptake
in absence of precipitation) Acidity Measure of
free hydrogen ion (more generally hydrated
hydrogen ion) content in solution pH Convenient
measure of acidity for dilute solution pH
-logH, where H is hydrogen ion conc. in
mole l-1
3
CHEMICAL SOURCES AND SINKS OF ACIDITY
Sources HNO3 ? NO3- H H2SO4 ? SO42-
2H Sinks NH3 H ? NH4 CaCO3(s) ? Ca2
CO32- CO32- 2H ? CO2(g) H2O
4
pH OF PURE WATER
- Consider pure water
- H2O(l) H OH-
Keq - Keq (H OH-)/H2O(l)
- H2O(l) is very much greater than H and OH-
- Define a new constant Kw KeqH2O(l)
- At 25 C, Kw 1 x 1014 M2
- H OH- 107 M i.e. pH 7
- Higher H ? lower pH
5
NORMAL ACIDITY
- Consider water in equilibrium with CO2 280 ppm
at 25 C - H2O(l) H OH-
Kw 1 x 10-14 M2 - CO2(g) H2CO3
KCH 3.1 x 10-2 M atm-1 - H2CO3 H HCO3- KC1
4.2 x 10-7 M - HCO3- H CO32-
KC2 5 x 10-11 M - ? H 1.9 x 10-6 M, i. e. pH 5.7
- Other natural acids (org. acids from biosphere,
HNO3 from lightning-generated NOx, H2SO4 from
sulfur gases emitted from biosphere and
volcanoes) and bases (NH3 from biosphere, CaCO3
from soil dust) and can also effect pH - ? Acid rain ? rain with pH below 5
6
ACID RAIN
Consider water in equilibrium with SO2 H2O(l)
H OH- Kw 1 x
10-14 M2 SO2(g) H2SO3
KSH H2SO3 H
HSO3- KS1 HSO3- H
SO32- KS2 In addition, acid
generation can occur by reactions involving
dissolved O3, H2O2, etc. H2SO3 O3 ? SO42- 2H
O2 kr1 HSO3- O3 ? H SO42-
O2 kr2 SO32- O3 ? SO42- O2
kr3
7
ACID RAIN
In addition, acid generation can occur by
reactions involving dissolved O3, H2O2,
etc. H2SO3 O3 ? SO42- 2H O2
kr1 HSO3- O3 ? H SO42- O2
kr2 SO32- O3 ? SO42- O2
kr3 HSO3- H2O2
SO2OOH- H2O Kr SO2OOH-
H ? 2H SO42- kr4
8
DEPENDENCE ON AQ.-PHASE OXIDATION PATHWAY ON pH
9
CHEMISTRY OF SULFUR COMPOUNDS IN THE TROPOSPHERE
10
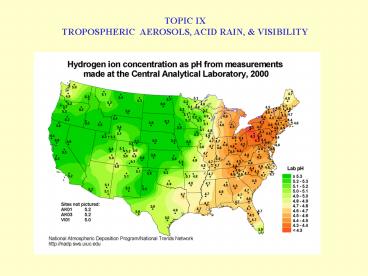
SELECTED ION CONC. MEASUREMENTS IN RAIN
11
SELECTED ION CONC. MEASUREMENTS IN RAIN
12
ATMOSPHERIC AEROSOLS
- Aerosols are solid or liquid particles or both
suspended in air - Ultra-fine aerosols 0.001-0.01 ?m
- Fine aerosols 0.01-1 ?m
- Coarse aerosols gt 1 ?m
- Importance of atmospheric aerosols
- heterogeneous chemistry
- air quality and human health
- visibility reduction
- acid deposition
- cloud formation
- climate change
13
FORMATION OF SULFATE AEROSOLS
14
CHEMICAL COMPOSITION OF TROPOSPHERIC AEROSOLS
15
ATTENUATION OF RADIATION BY AEROSOLS
- Aerosols can scatter and/or absorb solar and
infrared radn. - Scattering alters direction in which the
radiation propagates - Absorption removes energy from the radiation
field - Extinction (or attenuation)
- Sum of scattering and absorption
- Represents total effect on radiation
- Key parameters
- Wavelength of the incident radiation
- Size of the particles
- Chemical composition of the particles
16
SCATTERING OF RADIATION BY AEROSOLS
A reflection B refraction C refraction
internal refelection D diffraction
0.5 ?m light
17
DIRECT RADIATIVE FORCING OF ANTHRO. AEROSOLS
Fs incident solar flux, A planetary albedo
Solar flux absorbed per unit area on earths
surface Fs(1-A)/4 Add anthropogenic aerosol
layer of optical depth ? Direct radiative forcing
of aerosol layer -Fs?A/4 Global average ? is
about 0.1 ( 25 from anthro. aerosols) ? anthro.
aerosol optical depth is 0.025
18
DIRECT RADIATIVE FORCING OF ANTHRO. AEROSOLS
Radiation balance for aerosol layer Fd Fu Fs
- Ft Fs - Fse-? Fs - Fs (1 - ?) Fs? Albedo
of anthro. aerosol layer A Fu/Fs Let ?
Fu/(FdFu) typically ? 0.2) Fu ? (FdFu) ?
? Fs --gt A ? ? 5 x 10-3 for anthro. aerosols
19
DIRECT RADIATIVE FORCING OF ANTHRO. AEROSOLS
anthro. aerosol layer
natural aerosol layer earth surface
Horizontal overlap of reflective surfaces ?A is
less than A Total albedo
Assuming A ltlt 1 ?A AT - Ao A(1-Ao)2
20
DIRECT RADIATIVE FORCING OF SULFATE AEROSOLS
W m-2
21
THE IPCC THIRD ASSESSMENT
Direct effect of aerosols Partially compensates
for greenhouse gas forcing Indirect effect of
aerosols Effect on cloud albedo and lifetime ?
highly uncertain
22
AERSOLS AND VISIBILITY
- Clean (background) atmospheric conditions
- Light scattered and absorbed by background
aerosol - Visibility in absence of any aerosols 300 km
- Polluted atmospheric conditions
- Anthropogenic aerosols cause additional
attenuation of light - Significant degradation of visibility
23
VISIBILITY IN THE GREAT SMOKEY MOUNTAINS NATIONAL
PARK
Good Visibility Day Visual Range 100 miles
Bad Visibility Day Visual Range 20 miles
24
VISIBILITY DEGRADATION IN THE EASTERN U.S.
25
AEROSOL-GAS PHASE CHEMISTRY INTERACTIONS
- Formation of aerosol-precursor gases by
atmospheric oxidation (e.g, SO2 --gt H2SO4, NO2
--gt HNO3, hydrocarbons --gt low-volatility
secondary organic compounds) - Formation of oxidants in atmospheric oxdiation
cycles which take part in aqueous-phase chemistry
(e.g. H2O2) which can lead to increase in
accumulation mode mass - Formation of solid NH4NO3 by reaction between
gas-phase NH3 and HNO3 - Attenuation of solar radiation by aerosols
affecting photolysis - N2O5 hydrolysis