Vapor-Liquid Equilibrium using HYSYS - PowerPoint PPT Presentation
Title:
Vapor-Liquid Equilibrium using HYSYS
Description:
Its about application of HYSYS for flash calculations. an example from "Introduction to Chemical Engineering Thermodynamics by J.M.Smith" is used to simulate the results – PowerPoint PPT presentation
Number of Views:5186
Title: Vapor-Liquid Equilibrium using HYSYS
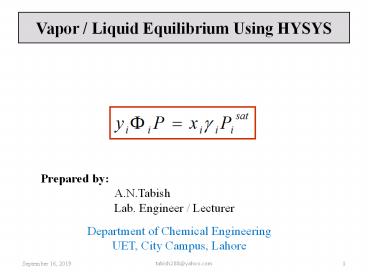
1
Vapor / Liquid Equilibrium Using HYSYS
Prepared by A.N.Tabish Lab. Engineer /
Lecturer
Department of Chemical Engineering UET, City
Campus, Lahore
2
Example 10.3
For the system methanol (1) / methyl acetate (2)
, the following equations provide a reasonable
correlation for the activity coefficient
Where,
In addition following equations provide vapor
pressures
Where T is in kelvin and the vapor pressure are
in kPa.
J.M.Smith, H.C.Van Ness and M.M.Abott (2005),
Introduction to Chemical Engineering
Thermodynamics, Ed. 7, pp. 359, McGraw Hill
3
CALCULATE
- P and y1, for T 318.15 K and x1 0.25
- P and x1, for T 318.15 K and y1 0.60
- T and y1, for P 101.33kPa and x1 0.85
- T and x1, for P 101.33kPa and y1 0.40
4
Solution Strategy
- Start a new case in HYSYS.
- Define components
- Select Thermodynamic model.
- Draw the PFD (material streams)
- Define
- Composition
- Vapor fraction
- Mole/mass flow rate
- Temperature / Pressure
- Determine BUBL/DEW Pressure/Temperature
- Determine composition using K-Value information
5
HYSYS
6
- Start a new case in HYSYS.
- Define components (Methanol Methyl Acetate).
7
- Select NRTL model.
8
- Draw the PFD (1 material streams)
9
- Vapor Pressure of Methanol
44.51kPa using Antoine Equation
- Vapor Pressure of Methyl Acetate
65.64kPa using Antoine Equation
10
BUBL Pressure
- P and y1, for T 318.15 K and x1 0.25
73.50 kPa in manual calculations
11
Vapor compositions
y1 0.282 y2 0.718
y1 0.278 y2 0.722
12
DEW Pressure
- P and x1, for T 318.15 K and y1 0.60
62.89 kPa in manual calculations
13
Liquid compositions
x1 0.8169 x2 0.1831
x1 0.8099 x2 0.1901
14
BUBL Temperature
- T and y1, for P 101.33kPa and x1 0.85
331.20K 58 oC in manual calculations
15
Vapor compositions
y1 0.670 y2 0.330
y1 0.671 y2 0.329
16
DEW Temperature
- T and x1, for P 101.33kPa and y1 0.40
326.70K 53.7 oC in manual calculations
17
Liquid compositions
x1 0.4602 x2 0.5398
x1 0.4517 x2 0.5483
18
VLE Plot
- Download registry files from
- http//people.clarkson.edu/wilcox/Design/EqPlots.
dll - http//people.clarkson.edu/wilcox/Design/EqPlots.
edf - and save them to your computer at some safe
place. - Create a case containing the components of
interest and choose a suitable fluid package. - Go to the Simulation Environment
- Go to Tools/Preferences/Extensions and Click on
Register an Extension. - Find EqPlots.dll and open it. This will remain
part of HYSYS or UniSim on your machine until it
is Unregistered. Close Preference. - Go to Flowsheet/Add Operation. Select the
Extensions category and Add Equilibrium Plots.
This should open the equilibrium plots extension. - For a Binary plot, click the Binary tab at the
bottom of the Equilibrium Plots menu, and select
the components and the type of plot desired. - For an XY plot, specify either T or P, and click
on Plot. - For a TXY plot, specify P. For a PXY plot,
specify T.
19
(No Transcript)
20
(No Transcript)
21
(No Transcript)
22
Example 10.4
- For a mixture of 10 mol- methane, 20 mol-
ethane, and 70 mol- propane at 50 (oF),
determin - The dew point pressure
- The bubble point pressure
J.M.Smith, H.C.Van Ness and M.M.Abott (2005),
Introduction to Chemical Engineering
Thermodynamics, Ed. 7, pp. 364, McGraw Hill
23
Home Work Task
- Assuming the validity of Raoult's law, do the
following calculations for the benzene
(l)/toluene (2) system
- For the system ethyl ethanoate (1) and n-heptane
(2) at 343.15 K (70"C)
BUBL P calculation for T 343.15 K (70C), x1
0.05. DEW P calculation for T 343.15 K (70C),
y1 0.05.
24
Home Work Task Contd.
- Make the following VLE calculations for the
methane (l) / ethylene (2) / ethane (3) system