New silanimine and NSiHM agostic complexes for coupling
New silanimine and NSiHM agostic complexes for coupling
New silanimine and NSi...H...M agostic complexes for coupling. with petroleum derived products ... metal complexes in a regio- and sterioselective manner. ... –
Title: New silanimine and NSiHM agostic complexes for coupling
1
New silanimine and NSiHM agostic complexes for
coupling with petroleum derived products
Georgii I. Nikonov, Department of Chemistry,
Brock University
Free silanimines are so highly reactive species
that when coordinated to transition metals they
still maintain a lot of their original
reactivity. Because free silanimnes are highly
unstable, their few known metal complexes were
prepared by a multistage pathway, which hampers
further development of this field to synthesis
and catalysis. Our research is focused on the
generation of reactive silanimine complexes by
the direct coupling of imido complexes with
silanes.
- The final goal is to achieve a catalytic
tri-component coupling of amines, silanes, and
petroleum derived unsaturated organic products
that would be mediated by transition metal
complexes in a regio- and sterioselective manner. - Our main accomplishments include
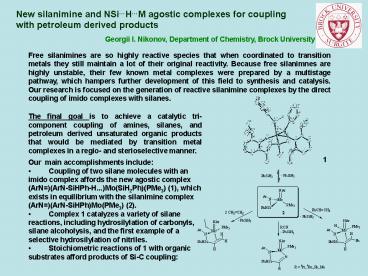
- Coupling of two silane molecules with an imido
complex affords the new agostic complex
(ArN)(ArN-SiHPh-H...)Mo(SiH2Ph)(PMe3) (1), which
exists in equilibrium with the silanimine complex
(ArN)(ArN-SiHPh)Mo(PMe3) (2). - Complex 1 catalyzes a variety of silane
reactions, including hydrosilylation of
carbonyls, silane alcoholysis, and the first
example of a selective hydrosilylation of
nitriles. - Stoichiometric reactions of 1 with organic
substrates afford products of Si-C coupling
1
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.