Tunable Ligands for RhodiumCatalyzed Hydroformylation - PowerPoint PPT Presentation
1 / 1
Title:
Tunable Ligands for RhodiumCatalyzed Hydroformylation
Description:
Hydroformylation is elegant in its simplicity an alkene reacts with CO and H2 ... compounds, including reactions involving sterically encumbered coupling partners. ... – PowerPoint PPT presentation
Number of Views:232
Avg rating:3.0/5.0
Title: Tunable Ligands for RhodiumCatalyzed Hydroformylation
1
Tunable Ligands for Rhodium-Catalyzed
Hydroformylation
Rhett C. Smith, Department of Chemistry, Clemson
University
Hydroformylation is elegant in its simplicity
an alkene reacts with CO and H2 to form a
synthetically versatile aldehyde with complete
atom economy. Even the simplest substrate, a
terminal olefin, produces linear (l) and branched
(b) aldehydes. The l-aldehyde is typically
desired, and we have focused on improving
regioselectivity through rational catalyst
design. The most active mononuclear
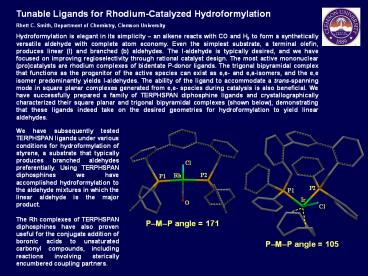
(pro)catalysts are rhodium complexes of bidentate
P-donor ligands. The trigonal bipyramidal complex
that functions as the progenitor of the active
species can exist as e,e- and e,a-isomers, and
the e,e isomer predominantly yields l-aldehydes.
The ability of the ligand to accommodate a
trans-spanning mode in square planar complexes
generated from e,e- species during catalysis is
also beneficial. We have successfully prepared a
family of TERPHSPAN diphosphine ligands and
crystallographically characterized their square
planar and trigonal bipyramidal complexes (shown
below), demonstrating that these ligands indeed
take on the desired geometries for
hydroformylation to yield linear aldehydes.
We have subsequently tested TERPHSPAN ligands
under various conditions for hydroformylation of
styrene, a substrate that typically produces
branched aldehydes preferentially. Using
TERPHSPAN diphosphines we have accomplished
hydroformylation to the aldehyde mixtures in
which the linear aldehyde is the major
product. The Rh complexes of TERPHSPAN
diphosphines have also proven useful for the
conjugate addition of boronic acids to
unsaturated carbonyl compounds, including
reactions involving sterically encumbered
coupling partners.
PMP angle 171
PMP angle 105