Putting Elements Together - PowerPoint PPT Presentation
1 / 9
Title:
Putting Elements Together
Description:
Putting Elements Together. If a sample of matter contains two or more elements ... The oppositely charged parts stick together. STRONG. WEAK ... – PowerPoint PPT presentation
Number of Views:65
Avg rating:3.0/5.0
Title: Putting Elements Together
1
Putting Elements Together
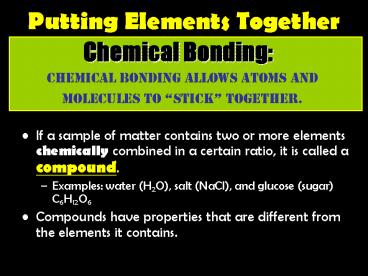
Chemical Bonding
Chemical bonding allows atoms and molecules to
stick together.
- If a sample of matter contains two or more
elements chemically combined in a certain ratio,
it is called a compound. - Examples water (H2O), salt (NaCl), and glucose
(sugar) C6H12O6 - Compounds have properties that are different from
the elements it contains.
2
Types of Chemical Bonding
STRONG
- Covalent Bonding Atoms share electrons.
- Ionic Bonding Atoms gain or lose electrons to
form charge particles called ions. Ions then
bond together because opposite charges attract. - Hydrogen Bonding Molecules stick together
because certain parts of them are charged. The
oppositely charged parts stick together.
WEAK
3
Covalent Bonding
Covalent Bonds form when atoms SHARE a pair of
electrons. This fills their outer valence shell.
Two or atoms held together by covalent bonds is a
molecule. Ex. H2O, O2, HCl, O3
They may share more than one bond between two
atoms
4
Water molecules attract by weak hydrogen bonds
Inside the Molecule covalent bonds
Cohesion like molecules stick together Adhesion
unlike molecules stick together
Water Water Water
5
Importance of Water
- Water molecules are polar
- Electronegativity
- Hydrophobic vs. hydrophilic
- Water molecules are cohesive
- Hydrogen bonds
- Water has a high specific heat
- Water can resist temp. change
- Important to cells regulates
- ecosystem climate
- Water is the universal solvent
- Dissolves/carries nutrients
- Cellular Transport
6
Strong cohesive forces between water molecules
create surface tension in water - droplets
Capillary action is created by the adhesion of
water to vessel walls and creates surface tension
(meniscus curve)
Special Properties of Water
7
- Knowing about the structure of atoms can help us
understand the functions of certain elements in
living things.
8
IONIC BONDING
how does salt form?
Na (1 valence e-) Cl (7 valence e-)
Na Cl-
- Ionic bonds form between metals and non-metals,
- Ionic compounds dissolve easily in water and
other polar solvents - In solution, ionic compounds easily conduct
electricity,
9
Covalent vs. Ionic Bonding