Chemical Equilibrium - PowerPoint PPT Presentation
1 / 84
Title:
Chemical Equilibrium
Description:
... and Hydrogen which produces methane (Tetrahydrogen monocarbide) and water. ... A. Changes in Concentration ... Use Le Ch telier's Principle to predict how ... – PowerPoint PPT presentation
Number of Views:222
Avg rating:3.0/5.0
Title: Chemical Equilibrium
1
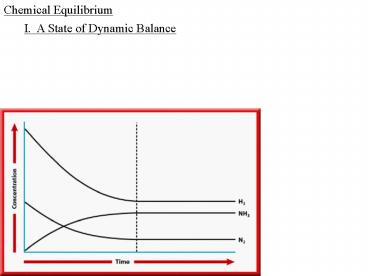
Chemical Equilibrium
I. A State of Dynamic Balance
2
Chemical Equilibrium
I. A State of Dynamic Balance
-when a ________ results in the almost ________
conversion of ________ to ________, the
________ is said to go to __________, but _____
_________ ___ ____ go to __________, most
_________ are __________
3
Chemical Equilibrium
I. A State of Dynamic Balance
-as soon as the ________ ________ begins, the
____________ of the _________ go _____, and the
_________ _____ goes _____ as the number of
__________ per unit ____ goes _____
4
Chemical Equilibrium
I. A State of Dynamic Balance
-as the _________ proceeds, the ____ of the
________ _________ continues to ________ and the
____ of the ________ ________ continues to
________ until the two _____ are _____, and the
system has reached a state of ________ __________
5
Chemical Equilibrium
I. A State of Dynamic Balance
-at ___________, the ____________ of the
________ and ________ are not _____, but
_______, because the ____ of _________ of the
________ is _____ to the ____ of _________ of
the ________
6
Chemical Equilibrium
II. Equilibrium Expressions and Constants
-while _____ chemical systems have little
tendency to _____, and _____ chemical systems
_____ readily and ___ to __________, _____
chemical systems reach a _____ of __________,
leaving varying amounts of ________ ____________
-in 1864, Norwegian chemists ______ and
_________ proposed the _______
___________________, which states, at a given
___________, a chemical system may reach a
_____ in which a particular _____ of _______
and _______ ____________ has a _______ value
7
Chemical Equilibrium
II. Equilibrium Expressions and Constants
-the _______ ________ for a _______ at
__________ can be written _______________________
_______, where __ and __ are ________, __ and
__ are ________, __, __, __, and __ are the
___________ in the ________ ________, and the
__________ _______ __________ is
-___________ ________ with ___ values __ __
contain more ________ than ________ at
___________, while __________ ________ with ___
values __ __ contain more ________ than
________ at __________
8
Chemical Equilibrium
II. Equilibrium Expressions and Constants
Write the equilibrium constant expression for the
homogeneous equilibrium for the synthesis of
ammonia from nitrogen and hydrogen.
9
Chemical Equilibrium
II. Equilibrium Expressions and Constants
Write the equilibrium constant expression for the
equilibrium for the synthesis of Hydrogen iodide
from iodine and hydrogen.
10
Chemical Equilibrium
II. Equilibrium Expressions and Constants
Write the equilibrium constant expression for the
equilibrium for the decomposition of Dinitrogen
tetroxide into Nitrogen dioxide.
11
Chemical Equilibrium
II. Equilibrium Expressions and Constants
Write the equilibrium constant expression for the
equilibrium for the reaction of Carbon monoxide
and Hydrogen which produces methane
(Tetrahydrogen monocarbide) and water.
12
Chemical Equilibrium
II. Equilibrium Expressions and Constants
Write the equilibrium constant expression for the
equilibrium for the decomposition of Dihydrogen
monosulfide into diatomic hydrogen and diatomic
sulfur.
13
Chemical Equilibrium
II. Equilibrium Expressions and Constants
-_________ in which all ________ and ________
are in the same ________ _____ are ____________,
but ________ with _________ and ________ in
_____ than ___ ________ _____ result in
_____________ _________
14
Chemical Equilibrium
II. Equilibrium Expressions and Constants
-since ______ and _____ ________ and ________
dont change ___________, (which is really their
______), if the ___________ remains ________,
then in the ___________ _______ __________ for a
____________ ___________, the ___________
________ only depends on the ______________ of
the ________ and ________ in the _______ state
of matter
15
Chemical Equilibrium
II. Equilibrium Expressions and Constants
Write the equilibrium constant expression for the
heterogeneous equilibrium for the decomposition
of Sodium Hydrogen carbonate into Sodium
carbonate, Carbon dioxide, and water.
16
Chemical Equilibrium
II. Equilibrium Expressions and Constants
Write the equilibrium constant expression for the
heterogeneous equilibrium for the decomposition
of Calcium carbonate into Calcium oxide and
Carbon dioxide.
17
Chemical Equilibrium
Name_________________
II. Equilibrium Expressions and Constants
Write the complete, balanced thermochemical
equation and equilibrium constant expression for
the homogeneous equilibrium for the reaction of
hydrazine (Tetrahydrogen dinitride) and Nitrogen
dioxide, which produces nitrogen and water.
18
Chemical Equilibrium
II. Equilibrium Expressions and Constants
Write the complete, balanced thermochemical
equation and equilibrium constant expression for
the homogeneous equilibrium for the reaction of
Sulfur trioxide and Carbon dioxide, which
produces Carbon disulfide and oxygen.
19
Chemical Equilibrium
II. Equilibrium Expressions and Constants
Write the complete, balanced thermochemical
equation and equilibrium constant expression for
the heterogeneous equilibrium for the reaction of
monatomic Sulfur and fluorine gas, which produces
Sulfur tetrafluoride gas and Sulfur hexafluoride
gas.
20
Chemical Equilibrium
II. Equilibrium Expressions and Constants
Write the complete, balanced thermochemical
equation and equilibrium constant expression for
the heterogeneous equilibrium for the reaction of
magnatite (Fe3O4) and hydrogen gas, which
produces iron and water vapor.
21
Chemical Equilibrium
II. Equilibrium Expressions and Constants
Write the equilibrium constant expression for the
homogeneous equilibrium for the synthesis of
ammonia and calculate the value of Keq when NH3
0.933 M, N2 0.533 M, and H2 1.600 M.
22
Chemical Equilibrium
II. Equilibrium Expressions and Constants
Write the equilibrium constant expression for the
homogeneous equilibrium for the decomposition of
Sulfur trioxide into Sulfur dioxide and oxygen
gas, and calculate the value of Keq when SO3
0.0160 M, SO2 0.00560 M, and O2 0.00210 M.
23
Chemical Equilibrium
III. Le Châteliers Principle
1. Hypothesis
2. Prediction
3. Gather Data
A. Safety
B. Procedure
24
Chemical Equilibrium
III. Le Châteliers Principle
3. Gather Data
B. Procedure
25
Chemical Equilibrium
III. Le Châteliers Principle
4. Analyze Data
5. Draw Conclusions
26
Chemical Equilibrium
III. Le Châteliers Principle
-________ that reach __________ instead of going
to __________ do not ________ as much
-in 1888, ________________________ discovered
that there are ways to _______ _________ in
order to make _________ more __________
-____________________ states that if a ______
(like a ______ in __________) is applied to a
system at __________, the system _____ in the
________ that _______ the _____
27
Chemical Equilibrium
III. Le Châteliers Principle
-stressors that cause a shift in equilibrium
A. Changes in Concentration
Write the equilibrium constant expression for the
equilibrium for the reaction of Carbon monoxide
and Hydrogen to produce methane and water. Then,
calculate the Keq value when CO 0.30000 M,
H2 0.10000 M, and CH4 0.05900 M, and
H2O 0.02000 M.
28
Chemical Equilibrium
III. Le Châteliers Principle
A. Changes in Concentration
-_________ the ____________ of ___ _________ the
_______ of _________ between ___ and ___,
_________ the _____ of the _______ _______
-the system responds to the ______ of the
addition of _______ by forming more _______ to
bring the system back into equilbrium
29
Chemical Equilibrium
III. Le Châteliers Principle
A. Changes in Concentration
30
Chemical Equilibrium
III. Le Châteliers Principle
A. Changes in Concentration
-_________ the ____________ of a ________ causes
__________ to _____ to the ____ to _______ the
____ of formation of ______
-_________ the ____________ of a ________ causes
__________ to _____ to the ____ to _______ the
____ of formation of ______
31
Chemical Equilibrium
III. Le Châteliers Principle
A. Changes in Concentration
Predict what should happen to the following
equilibrium if hydrogen bonding due to the
addition of acetone binds water and effectively
removes it from the products.
32
Chemical Equilibrium
III. Le Châteliers Principle
-stressors that cause a shift in equilibrium
A. Changes in Volume
-_________ the ______ of the _______ container,
according to ______, ________ the ________,
which in turn ________ the _____ of _________
between the ________ of the ________, _________
the _____ of the ________ _______
-the _____ in the _________ causes the _____ on
the system to be _______ as for every __ _____
of _______ _______ _________, only __ _____ of
_______ _______ are _________, which,
according to ________, occupies __ the ______,
which _________ the ________
33
Chemical Equilibrium
III. Le Châteliers Principle
-stressors that cause a shift in equilibrium
Use Le Châteliers Principle to predict how each
of these changes would affect the ammonia
equilibrium system.
a. removing hydrogen from the system
__________________________
b. adding ammonia to the system
_______________________________
34
Chemical Equilibrium
III. Le Châteliers Principle
-stressors that cause a shift in equilibrium
Use Le Châteliers Principle to predict how each
of these changes would affect the ammonia
equilibrium system.
1N2 (g)
3H2(g)
2NH3(g)
c. adding hydrogen to the system
_______________________________
35
Chemical Equilibrium
III. Le Châteliers Principle
-stressors that cause a shift in equilibrium
How would decreasing the volume of the reaction
container affect each of these equilibria?
a.
_________________________
1H2 (g)
1Cl2(g)
2HCl(g)
b.
_____________________________
2NOBr(g)
1Br2(g)
2NO(g)
c.
_________________________
36
Chemical Equilibrium
III. Le Châteliers Principle
-stressors that cause a shift in equilibrium
A. Changes in Temperature
-while _______ in _____________ and ________ in
_______ cause ______ in _________, they ___ ___
_______ the __________ _______, but a ______ in
___________ causes ______ in both the
__________ ________ and the __________ _______
37
Chemical Equilibrium
III. Le Châteliers Principle
-stressors that cause a shift in equilibrium
A. Changes in Temperature
-since the _______ for making _______ has a
_______ ____, the ________ _______ is
_________, and the _______ _______ is
__________, so ____ can be thought of as a
_______ in the ________ _______ and a _______
in the _______ _______
38
Chemical Equilibrium
III. Le Châteliers Principle
-stressors that cause a shift in equilibrium
A. Changes in Temperature
-_________ the __________ is like _______ more
_______ to the _______ in which _____ acts as a
_______ and is _____ ___, in this case, the
__________ _______ _______
-__________ shifts to the _____, _________ the
___________ of _______ because _______ is a
_______ in the _______ _______
39
Chemical Equilibrium
III. Le Châteliers Principle
-stressors that cause a shift in equilibrium
A. Changes in Temperature
-_________ the __________ is like ________
_______ from the _______ in which _____ acts as
a _______, in this case, the __________
_______ _______
-__________ shifts to the _____, _________ the
___________ of _______ because _______ is a
_______ in the _______ _______
40
Chemical Equilibrium
III. Le Châteliers Principle
-stressors that cause a shift in equilibrium
In the following equilibrium, would you raise or
lower the temperature to get the following
results?
a. increase the amount of CH3CHO_________________
_____________
b. decrease the amount of C2H2
________________________________
41
Chemical Equilibrium
III. Le Châteliers Principle
-stressors that cause a shift in equilibrium
In the following equilibrium, would you raise or
lower the temperature to get the following
results?
1C2H2 (g)
1H2O(g)
1CH3CHO(g)
?H0 -151 kJ
c. increase the amount of H2O ___________________
______________
42
Chemical Equilibrium
III. Le Châteliers Principle
-stressors that cause a shift in equilibrium
In the following equilibrium, what effect does
changing the volume of the reaction vessel have?
__________________________________________________
__________________________________________________
__________________________________________________
________________________
In the following equilibrium, what effect does
simultaneously increasing the temperature and the
pressure have?
__________________________________________________
__________________________________________________
__________________________________________________
________________________
43
Chemical Equilibrium
III. Le Châteliers Principle
1. Hypothesis
2. Prediction
3. Gather Data
A. Safety
44
Chemical Equilibrium
III. Le Châteliers Principle
3. Gather Data
B. Procedure
45
Chemical Equilibrium
III. Le Châteliers Principle
3. Gather Data
B. Procedure
4. Analyze Data
A. The equation for the reversible reaction in
this experiment is
46
Chemical Equilibrium
III. Le Châteliers Principle
4. Analyze Data
A. Use the equation to explain the colors of the
solution in steps 1, 2, and 3
47
Chemical Equilibrium
III. Le Châteliers Principle
4. Analyze Data
B. Explain how the equilibrium shifts when heat
energy is added or removed.
5. Draw Conclusions
48
Chemical Equilibrium
IV. Using Equilibrium Constants
-when a ________ has a _____ ___, the __________
_______ contains _____ ________ than ________
at __________
-when a ________ has a _____ ___, the __________
_______ contains _____ ________ than ________
at __________
A. Calculating Equilibrium Concentrations
-__________ ________ can also be used to
________ the __________ ____________ of
any ________ in the _______
49
Chemical Equilibrium
IV. Using Equilibrium Constants
A. Calculating Equilibrium Concentrations
At 1200 K, the Keq for the following reaction
equals 3.933. What is the concentration of the
methane produced, if CO 0.850 M, H2 1.333
M, and H2O 0.286 M?
50
Chemical Equilibrium
IV. Using Equilibrium Constants
A. Calculating Equilibrium Concentrations
At 1405 K, the Keq for the following reaction
equals 2.27 x 10-3. What is the concentration of
the Hydrogen gas produced, if S2 0.0540 M,
and H2S 0.184 M?
51
Chemical Equilibrium
IV. Using Equilibrium Constants
A. Calculating Equilibrium Concentrations
If Keq for the following reaction equals 10.5,
what is the equilibrium concentration of Carbon
monoxide, if H2 0.933 M, and CH3OH 1.32 M?
52
Chemical Equilibrium
IV. Using Equilibrium Constants
A. Calculating Equilibrium Concentrations from
Initial Concentrations Using ICE (Initial,
Change, Equilibrium)
If the Keq for the following reaction equals
64.0, what are the equilibrium concentrations of
I2, H2, and HI, if I20 0.200 M, H20 0.200
M and HI 0.000 M?
53
Chemical Equilibrium
IV. Using Equilibrium Constants
A. Calculating Equilibrium Concentrations from
Initial Concentrations Using ICE (Initial,
Change, Equilibrium)
1H2 (g)
2HI(g)
1I2(g)
H2
I2
HI
Initial
0.200
0.200
0.000
Change
-1x
-1x
2x
Equilibrium
0.200 - 1x
0.200 - 1x
2x
54
Chemical Equilibrium
IV. Using Equilibrium Constants
A. Calculating Equilibrium Concentrations from
Initial Concentrations Using ICE (Initial,
Change, Equilibrium)
If the Keq for the following reaction equals
16.0, what are the equilibrium concentrations of
PCl3, Cl2, and PCl5, if PCl50 1.00 M?
55
Chemical Equilibrium
IV. Using Equilibrium Constants
A. Calculating Equilibrium Concentrations from
Initial Concentrations Using ICE (Initial,
Change, Equilibrium)
1Cl2 (g)
1PCl5(g)
1PCl3(g)
PCl3
Cl2
PCl5
Initial
0.00
0.00
1.00
Change
1x
1x
-1x
Equilibrium
0.00 1x
0.00 1x
1.00 1x
56
Chemical Equilibrium
IV. Using Equilibrium Constants
A. Calculating Equilibrium Concentrations from
Initial Concentrations Using ICE (Initial,
Change, Equilibrium)
57
Chemical Equilibrium
IV. Using Equilibrium Constants
A. Calculating Equilibrium Concentrations from
Initial Concentrations Using ICE (Initial,
Change, Equilibrium)
If the Keq for the following reaction equals
0.680, what are the equilibrium concentrations of
COCl2, CO, and Cl2, if CO0 0.500 M and Cl20
1.00 M?
58
Chemical Equilibrium
IV. Using Equilibrium Constants
A. Calculating Equilibrium Concentrations from
Initial Concentrations Using ICE (Initial,
Change, Equilibrium)
1Cl2 (g)
1CO(g)
1COCl2 (g)
COCl2
CO
Cl2
Initial
0.00
0.500
1.00
Change
1x
-1x
-1x
Equilibrium
0.00 1x
0.500 - 1x
1.00 1x
59
Chemical Equilibrium
IV. Using Equilibrium Constants
A. Calculating Equilibrium Concentrations from
Initial Concentrations Using ICE (Initial,
Change, Equilibrium)
60
Chemical Equilibrium
Name_________________
IV. Using Equilibrium Constants
A. Calculating Equilibrium Concentrations from
Initial Concentrations Using ICE (Initial,
Change, Equilibrium)
If the Keq for the following reaction equals
36.0, what are the equilibrium concentrations of
H2, Br2, and HBr, if H20 0.250 M and Br20
0.250 M?
H2
Br2
HBr
Initial
Change
Equilibrium
61
Chemical Equilibrium
IV. Using Equilibrium Constants
A. Calculating Equilibrium Concentrations from
Initial Concentrations Using ICE (Initial,
Change, Equilibrium)
H2
Br2
HBr
Initial
Change
Equilibrium
62
Chemical Equilibrium
IV. Using Equilibrium Constants
A. Calculating Equilibrium Concentrations from
Initial Concentrations Using ICE (Initial,
Change, Equilibrium)
63
Chemical Equilibrium
IV. Using Equilibrium Constants
A. Calculating Equilibrium Concentrations from
Initial Concentrations Using ICE (Initial,
Change, Equilibrium)
If the Keq for the following reaction equals
20.0, what are the equilibrium concentrations of
H2, Cl2, and HCl, if H20 1.00 M and Cl20
2.00 M?
H2
Cl2
HCl
Initial
Change
Equilibrium
64
Chemical Equilibrium
IV. Using Equilibrium Constants
A. Calculating Equilibrium Concentrations from
Initial Concentrations Using ICE (Initial,
Change, Equilibrium)
H2
Cl2
HCl
Initial
Change
Equilibrium
65
Chemical Equilibrium
IV. Using Equilibrium Constants
A. Calculating Equilibrium Concentrations from
Initial Concentrations Using ICE (Initial,
Change, Equilibrium)
66
Chemical Equilibrium
V. Solubility Equilibria
-like a few _________ _________ that go to
_________, upon __________, some ______
__________ _________ completely into _____
-some _____ __________, however, are only
________ _______, and quickly reach a ________
__________
67
Chemical Equilibrium
V. Solubility Equilibria
-in the __________ _______ __________, ______
______ is a _____, so the _______ is _______,
and can be combined with the ___ value to form
the ________ _______ _______
Write the solubility constant expression for the
following solubility equilibrium
68
Chemical Equilibrium
V. Solubility Equilibria
A. Calculating Solubilities from
Solubility Product Constants
What is the solubility, in M, of Silver iodide at
298 K?
69
Chemical Equilibrium
V. Solubility Equilibria
A. Calculating Solubilities from
Solubility Product Constants
What is the solubility, in M, of Copper(II)
carbonate at 298 K?
70
Chemical Equilibrium
V. Solubility Equilibria
B. Calculating Ion Concentration from Ksp
What is OH- at 298 K in a saturated solution of
Mg(OH)2 at equilibrium?
71
Chemical Equilibrium
V. Solubility Equilibria
B. Calculating Ion Concentration from Ksp
What is Ag at 298 K in a saturated solution of
AgBr at equilibrium?
72
Chemical Equilibrium
V. Solubility Equilibria
B. Calculating Ion Concentration from Ksp
What is F- at 298 K in a saturated solution of
CaF2 at equilibrium?
73
Chemical Equilibrium
V. Solubility Equilibria
C. Predicting Precipitates
-besides being used to calculate the _________
of an _____ _________ and the ___________ of
____ in a _________ _______, ___ values can be
used to _______ if a _________ will form if ___
_____ __________ are mixed
Predict whether PbCl2 will form as a precipitate
if 100 mL of 0.0100 M NaCl is added to 100 mL of
0.0200 M Pb(NO3)2
-the ____________ of the ______ ________ allow
you to _______ the ____________ of ____ and ___
ions in the _____ _________, which when
_________ together, determine the ___ _______,
or ___
74
Chemical Equilibrium
V. Solubility Equilibria
C. Predicting Precipitates
Predict whether PbCl2 will form as a precipitate
if 100 mL of 0.0100 M NaCl is added to 100 mL of
0.0200 M Pb(NO3)2
75
Chemical Equilibrium
V. Solubility Equilibria
C. Predicting Precipitates
-if the ___ is ___ the ___, the _______ is
__________, and a _________ ____ ___ ____, and
if the ___ is ___ the ___, the _______ is
_________ and ___ ______ will occur, but if ___
is ___ the ___, a __________ will form, reducing
the ___ ___________ until ___ ___ ___, and the
system arrives at __________ and the _______
becomes ________
76
Chemical Equilibrium
V. Solubility Equilibria
C. Predicting Precipitates
Predict whether Ag2SO4 will form as a precipitate
if 500 mL of 0.010 M AgNO3 is added to 500 mL of
0.25 M K2SO4
77
Chemical Equilibrium
V. Solubility Equilibria
C. Predicting Precipitates
Predict whether a precipitate will form if 200 mL
of 0.20 M MgCl2 is added to 200 mL of 0.0025 M
NaOH
78
Chemical Equilibrium
V. Solubility Equilibria
D. Common Ion Effect
-the ________ of _______ in _____ is ________
mol/L, which means that you can ________
________ of _______ in ____ L of _____ _____,
but _________ of _______ will ____ _______ in
____ L of a ______ solution of _______, because
of the ________ ___ ______
-since the _______ of the ____________ of both
____ is _____ to a _______, (the _________
_______ _______), if _______ goes __, _____ must
go _____
79
Chemical Equilibrium
V. Solubility Equilibria
D. Common Ion Effect
-adding a _______ to an __________ that
contains a ________ ___ _______ the ________ of
a _________ containing that ___, or, according
to _____________ ________, stresses the
__________ and causes the _______ to _____ the
__________ in the _______ that _______ the
______
80
Chemical Equilibrium
Chemical Equilibrium
V. Solubility Equilibria
1. Hypothesis
2. Prediction
3. Gather Data
A. Safety
B. Procedure
1. Using a pipette, place 10 drops of AgNO3
solution into test well A1 of a 20-well
microplate. Place 10 more drops of the
same solution in test well A2.
81
Chemical Equilibrium
V. Solubility Equilibria
3. Gather Data
B. Procedure
2. Add 10 drops of NaCl solution to both test
well A1 and test well A2. Record
observations___________________
3. To test well A2 only, add 10 drops of Na2S
solution. Record observations________________
______________
4. Compare the contents of test wells A1 and A2.
Record observations_________________________
____________
82
Chemical Equilibrium
V. Solubility Equilibria
4. Analyze Data
A. Write the complete thermochemical equation
for the reaction that occurred in Step 2.
B. Write the net ionic equation for the reaction
in Step 2.
C. Write the equation for the solubility
equilibrium that was established in test
wells A1 and A2 during Step 2.
D. Write the solubility constant expression for
the equilibrium established in test wells
A1 and A2 during Step 2.
E. Write the equation for the solubility
equilibrium that was established in test
well A2 during Step 4.
83
Chemical Equilibrium
V. Solubility Equilibria
4. Analyze Data
F. Match the chemical formula of each
precipitate with its color.
G. Compare the two Ksp values for the two
precipitates. Infer which is the more
soluble.
H. Use Le Châteliers Principle to explain how
the addition of Na2S in Step 4 affected the
equilibrium in test well A2.
84
Chemical Equilibrium
V. Solubility Equilibria
4. Analyze Data
I. Calculate the molar solubilities of both
precipitates in the experiment. Which of the
precipitates is more soluble?