Meat Proteins - PowerPoint PPT Presentation
1 / 47
Title: Meat Proteins
1
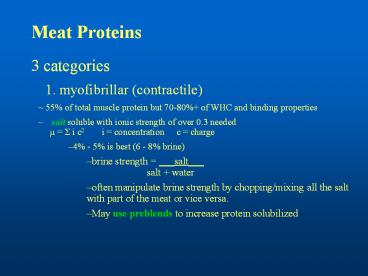
Meat Proteins
- 3 categories
- 1. myofibrillar (contractile)
- 55 of total muscle protein but 70-80 of WHC
and binding properties - salt soluble with ionic strength of over 0.3
neededµ ? i c2 i concentration c
charge - 4 - 5 is best (6 - 8 brine)
- brine strength ___salt___ salt
water - often manipulate brine strength by
chopping/mixing all the salt with part of the
meat or vice versa. - May use preblends to increase protein solubilized
2
1. myofibrillar (contractile)
- absolutely critical to processing properties
- i.e. bind values (WHC, fat binding, etc.)
- emulsion/batter products such as frankfurters -
will cover later - heat-set gelation which controls binding and
texture - hams, emulsion/batters, all cooked products
3
1. myofibrillar proteins are composed of
myosin 55
actin
40 - 45
troponin
tropomyosin
desmin, synemin, ? actinin, nebulin and numerous
structural proteins
1 -5
4
Myosin is generally considered the singly most
important because
- Long filamentous molecule (similar to a 1 inch
garden hose that is 8 feet long) - amino acid composition gives highly-charged,
polar molecule - present in large quantity in lean muscle
5
Other proteins are also important
- Many are charged, polar molecules
- structural proteins can have a large influence on
release of myosin/actin and opening protein
structure to water. - i.e. desmin degradation in aging can increase WHC
6
2. Stromal proteins (connective tissue)
- 10 - 15 of total muscle protein
- primarily collagen
- most abundant protein in animal body (20 -25 of
total body protein) - skin, sinews, tendons, etc. - designed to transmit force and hold things
together, therefore these proteins are generally
tough and inert - also - content will vary
according to muscle function - increased crosslinking as animal age increases
toughness and a major cause for sausage and
ground beef industries
7
2. Stromal proteins (connective tissue)
- Not very valuable in processed meats --- has
little binding ability - will shrink when heated to 140oF (with moisture)
and convert to gelatin at 160oF - 180oF - but
- if heated when dry --- collagen becomes very
hard and impermeable --- important to handling of
collagen and/or natural casings - collagen is highly resistant to enzymes so enzyme
tenderizers are generally ineffective
8
2. Stromal proteins (connective tissue)
- Unique protein with 33 glycine and
10 hydroxyproline - therefore very nonpolar noncharged molecules
- isoelectric point is about pH 7.2 - by far the only protein to contain large amounts
of hydroxyproline - - therefore
-hydroxyproline measurement is the most common
method used to determine collagen content in meat
9
2. Stromal proteins (connective tissue)
- Collagen is used to make gelatin, contact lenses,
pharmaceuticals, etc. - - and - regenerated sausage casings
10
(No Transcript)
11
2. Stromal proteins (connective tissue)
- generally considered a problem in processed meats
and high collagen meats often limited to 15 - 25
maximum - - however - chopped, ground, powdered
collagen which can be dispersed, can be useful in
forming a gel when heated and also in retaining
water and fat
12
3. Sarcoplasmic proteins (water soluble,
intracellular fluid)
- 30 of total muscle protein ( 20 of binding
ability) - isoelectric points generally between pH 6 - pH 7
- hundreds of enzymes in cells for energy, growth,
etc. - most are relatively low molecular weight (small)
proteins
13
Importance of sarcoplasmic proteins
- 1. Enzyme activity
- calpain - tenderization
- postmortem glycolysis
- pH change
- potential flavor contributions from
protein hydrolysis ? hydrolized proteins - 2. Color
- myoglobin
- responsible for all meat color variations so a
good understanding is critical in meat processing
14
Myoglobin
- conjugated protein
- consists of a typical amino acid protein chain
- - and -a non-protein heme molecule
15
(No Transcript)
16
(No Transcript)
17
Heme portion
- Responsible for all color
- Protein portion
- colorless - but -
- is important to heme stability and affects color
indirectly - free heme oxidizes to brown quickly
18
Heme is attached to the protein by a histidine
amino acid and the 5th bond from iron
- 6th bond is relatively free to bind oxygen,
nitric oxide, carbon monoxide or other compounds
that affect color
19
A second histidine on the protein chain --- on
the other side of the heme is important to
stability of fresh meat color.
- Not important to cured color
20
(No Transcript)
21
So --- what controls meat color?
- 1. Myoglobin concentration
- color intensity
- poultry white muscle .05 mg/g
- chicken thigh 1.8-2.0 mg/g
- turkey thigh 2.5-3.0 mg/g
- pork, veal 1.0-3.0 mg/g
- beef 4.0-10.0 mg/g
- old beef 15.0-20.0 mg/g
- mechanically separatedmeat 0.08-3.0 mg/g
22
2. Chemistry
- Fresh meat color comes from
- myoglobin - Fe - no ligand? (purple)
- oxymyoglobin - Fe - oxygen attached at 6th
position on heme (cherry red) - carboxymyoglobin - Fe carbon monoxide at 6th
position (cherry red) - metmyoglobin - Fe - no ligand (brown)
- therefore oxidation state of Fe(2,3) and
attached ligand (O2, CO, etc.) determine color
23
Four major chemical factors that affect the
pigment forms in fresh meat --- Fresh color -
- 1. Postmortem age/freshness
- myoglobin was biologically designed to hold
oxygen, then release it for energy metabolism So
- myoglobin binds oxygen somewhat temporarily ---
but must be in reduced Fe to do that
24
Reducing capacity of muscle keeps iron converted
from Fe to Fe and improves fresh color. ---
depends on active reducing enzymes
- Fresh meat is alive
- uses O2 ? CO2 to gain some energy to keep
enzymes and reducing ability active
25
As long as meat is fresh enough to keep Fe
reduced, color is desirable (purple red)
- With age, reducing capacity is lost and
metmyoglobin (brown) begins to predominate
26
2. pH
- High pH favors pigment reduction and fresh color
stability - pH is very interactive with and dependent on..
27
3. temperature
- Lower temperature is better
- Example a study of oxymyoglobin half-life
(time required to lose 1/2 of the oxymyoglobin
present) in solution gave the following --- - pH 5, 0oC --- 5 days
- pH 5, 25oC --- 3 hours
- pH 9, 25oC --- 7 days
- pH 9, 0oC --- 12 months
28
pH is also a factor in cooked color and can
affect visual appearance of doneness
- High pH
- retains pink/red color at high temperatures
pinking of cooked products - low pH
- may result in browning at low temperatures that
are microbiologically unsafe premature browning
29
4. Oxygen pressure
- atmospheric oxygen pressure gives oxygen binding
by myoglobin and red bloom from oxygenation of
pigment - low oxygen pressure results in oxidation of
pigment to metmyoglobin - thus a poor vacuum package can result in
discoloration of fresh meat - gives color gradient from surface to inside on
fresh meat
30
(No Transcript)
31
(No Transcript)
32
Oxidation is also accelerated by salt ---
- May cause disruption of protein and destabilizing
the heme/histidine arrangement - may suppress reducing enzymes
- will also result in rancid off-flavors if not
compensated correctly
33
Factors controlling cured color
- Must attach nitric oxide (NO) to heme to achieve
cured color - affinity of NO for heme is 100 times as great
as is oxygen - therefore NO will react with reduced or
oxidized heme - key to cured meat color is formation of NO in meat
34
(No Transcript)
35
To maximize cured color
1. Provide sufficient nitrite - NO2-
- NO2- reducing enzymes ? NO (relatively slow)
- 2 NO2- 2H(acid) ? 2HONO ? NO NO3- 2H
- NO2- Fe (heme) ? Fe NO
- these are three natural reactions of nitrite in
meat that are significant sources of NO for color
development
36
2. Accelerate NO production from NO2-
- increase acidity (H)
- pH of 5.4 will develop cured color twice as fast
as pH 5.7 --- may add acid (sodium acid
pyrophoshate, glucono delta lactone, citric acid) - increase reducing capacity
- add sodium erythorbate or sodium ascorbate
- permitted as curing accelerators
37
3. Heating / cooking
- Cured pigment is stabilized by heating over
130oF - 140oF - believed to remove heme from protein chain ---
giving free heme and attaches a second NO group
to the heme --- resulting in two attached NO
groups on either side of the heme
38
(No Transcript)
39
Cured meat color will fade
Especially in presence of light and oxygen
- NO
- Fe Fe NO
NO2- (nitrite)
O2 - NO
- NO2
(nitrogen dioxide gas) - therefore vacuum systems and vacuum packaging
are essential
light
40
Common color problems / questions
- 1. Iridescent blue-green sheen on roast beef and
ham slices - microbiological (hydrogen peroxide) or chemical
(nitrite burn, sanitizers) --- least likely - surface fat/oil film --- unlikely
- irregular muscle fiber surface from
non-perpendicular slicing angle
41
2. Pigment oxidation - gray, green etc.
- Light, oxygen exposure for cured meat
- nitrite burn - due to abnormally high nitrite
concentration - bacterial - some produce hydrogen peroxide (H2O2)
- rancid fat - radicals may oxidize heme
- close relationship between rancidity and color
because oxidized heme iron can induce rancidity
42
3. Pinking in uncured meat
- high pH
- nitrite, nitrate contamination from water,
vegetables, etc. - carbon monoxide in the environment
- transportation truck exhaust
- nitrogen oxide gases from cooking
- i.e. Hickory Park
43
4. Poor cured color development
- pH
- phosphates will slow color formation
- heating rate
- too fast will not allow adequate development
- too low nitrite concentration
- too low reductant level (ascorbate, erythorbate)
44
5. Smoke color variation
- Surface moisture is critical
- wet - streaked, uneven, - even black if very
excessive - dry - little or no color
45
6. Browning of fresh sausage
- Salt favors oxidation
- encapsulated salt
- meat freshness is important
- pre-rigor meat has best color
46
For cured color
- Maximize production of NO from NO2-
- but need to retain a small amount of NO2-
( 10-20 ppm) in the product for color stability
during distribution and display (especially
retail lighting in cases, etc.)
47
(No Transcript)