Nucleation Theory
Title:
Nucleation Theory
Description:
Transformation liquid (l) to cluster/embryo (Cr) Affinity: ... Interfacial Tension: Li2O.SiO2 Crystal Nucleation', Weinberg, Zabotto and Manrich, Physics and ... –
Number of Views:1354
Avg rating:3.0/5.0
Title: Nucleation Theory
1
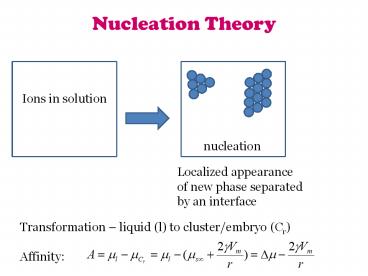
Nucleation Theory
Ions in solution
nucleation
Localized appearance of new phase separated by
an interface
Transformation liquid (l) to cluster/embryo
(Cr) Affinity
2
Nucleation Theory
Homogeneous Nucleation Supersaturated solution
assat gt asat
Supersaturation, DGvgt0
Gibbs Free Energy
DGv
Csat
Cssat
C
Concentration
3
Nucleation Theory
Volume Surface energy energy
r
4
Nucleation Theory
Volume Surface energy
energy
DG occurs when d(DG)/dr0
r occurs when d(DG)/dr0 embryos rltr clusters
rgtr
DGgtkT metastable nucleation DGltkT unstable
5
Nucleation Theory
Temperature Effect
DG
g
r
T
DG
DG
r
T1
T2
T3
6
Nucleation Theory
Time Effect
Nucleation rate
Cmax
nucleation
RNnPG
n is the of nucleation centers
embryos
growth
Cmin
P is the probability of a thermal fluctuation
with at least DG energy
Concentration
Csat
G is the surface atom jump frequency
I
II
III
time
7
Nucleation Theory
Nucleation Rate
Growth Rate
Diffusion
Surface Reaction
layer by layer
C
polynuclear
Csurf
8
Nucleation Theory
Nucleation Rate
Diffusion
Surface Reaction
layer by layer
polynuclear
layer by layer
polynuclear
9
Nucleation Theory
Nucleation Rate
layer by layer small
polynuclear medium
diffusion large
10
Nucleation Theory
Heterogeneous
Ions in solution
Seed particles
nucleation
11
Nucleation Theory
Heterogeneous
Qgt0
Q0 No barrier
Q180 homogeneous
12
Nucleation Theory
Heterogeneous Growth Mechanisms
Island or Volmer-Weber qgt0
Layer or Frank- van der Merwe q0
Island-Layer or Stranski-Krastonov
13
Nucleation Theory
- Works well for nonpolar liquids, not as well for
polar liquids - Steady-state nucleation rates often much too low
- Activation energy for surface jumps
- Surface energy used from flat interface,
curvature changes - this, and surface energy a function of r
Classical Nucleation Theory with a Size
Dependent Interfacial Tension Li2O.SiO2 Crystal
Nucleation, Weinberg, Zabotto and Manrich,
Physics and Chemistry of Glasses, 33 (3), pp.
99-102, 1992.