Ozone - PowerPoint PPT Presentation
1 / 14
Title:
Ozone
Description:
Some marine organisms with CaCO3 shells. Decreased pH increases CaCO3 solubility ... Pteropod (aragonite) shell in undersaturated waters ... – PowerPoint PPT presentation
Number of Views:48
Avg rating:3.0/5.0
Title: Ozone
1
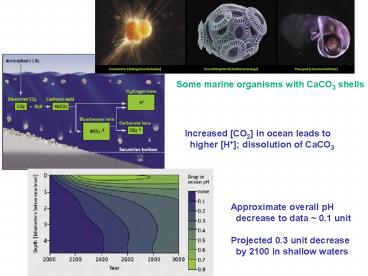
Some marine organisms with CaCO3 shells
Increased CO2 in ocean leads to higher H
dissolution of CaCO3
Approximate overall pH decrease to data 0.1
unit Projected 0.3 unit decrease by 2100 in
shallow waters
2
Decreased pH increases CaCO3 solubility Warmer
temperature increases CaCO3 solubility Bleaching
also occurs at warmer temperature
3
aragonite shell magnesium calcite shell
- Aragonite and calcite different crystal forms of
CaCO3 - Some calcite minerals substitute Mg2 for some
Ca2 - Aragonite and magnesium calcite are more soluble
than calcite, - and are more susceptible to CO2-generated
acidification
4
Aragonite oversaturation and undersaturation
Pteropod (aragonite) shell in undersaturated
waters
normal shell
5
(No Transcript)
6
Redox reactions in organic molecules
-Oxidation states of carbon counting owned
electrons provides a means of determining
whether a reaction oxidizes or reduces the
compound -The C in methane is least oxidized
that in carbon dioxide is most oxidized -Biomass
synthesis (carbon cycle) can also be considered a
redox reaction
7
Biological oxygen demand BOD
Biological oxygen demand of milligrams of
O2 required to oxidize the amount of organic
carbon in 1 L H2O Unpolluted water BOD 0.7
mg O2/liter Sewage - BOD 200 mg
O2/liter High BOD threatens aerobic aquatic
life Solubility of O2 in water 9 mg/L at 20C.
Set by Henrys Law, with KH 1.3 x 10-3
M/atm. PO2 0.21 atm. Solubility is higher
at lower temperature (thermal pollution). If
BOD exceeds solubility, then there may be no
remaining free O2 to sustain aerobic aquatic
life. Depends on the relative rates of oxygen
dissolution in to the water, and the speed of the
microbial metabolism that is consuming the O2.
8
Redox scale in biogeochemistry
O2 is the most powerful oxidizing agent
(oxidant) Creation of O2 by photosynthesis
greatly enabled biological metabolism in
cells, since the free energy change is very
high Without O2, though, redox reactions still
occur, to exploit anaerobic environments
The redox reactions, giving negative free-energy
changes, are mediated biologically, mainly by
single-celled bacteria and archaea
9
Redox chemistry in natural waters
Aerobic organisms CH2O O2 ? CO2 H2O
epilimnion - oxidized
hypolimnion - reduced no contact with air O2
fully consumed
Anaerobic bacteria 2 CH2O ? CH4 CO2
10
Reactions on the early Earth
½ O2 2 H 2 e- ? H2O E 0.816 V 2 Fe2 ?
2 Fe3 2 e- E -0.771 V
- First organisms exploited anaerobic redox
chemistry - Development of (oxygenic) photosynthesis produced
oxygen - Fossil fuel burial helped drive O2 accumulation
- The O2 was first used to drive the oxidation of
Fe2 to form insoluble Fe2O3 - bands in silicate rock (oceans) 2.5 3
billion years ago - Next, O2 attacked oxidizable minerals on land
- FeS2 O2 H2O ? Fe(OH)3 H2SO4 (red
bands, 2 billion years old) - Only after oceanic Fe2 and reduced sulfides were
oxidized, was - it possible for O2 to build up in the
atmosphere - 98 of all O2 ever produced is locked up in
oxidized minerals - Current O2 levels (21) achieved 400 million
years ago
11
Energy-use classification of living organisms
Energy obtained using organic or inorganic
redox reactions under anaerobic conditions
12
Redox scale in biogeochemistry
0.81 V 0.75 V
O2 is the most powerful oxidizing agent
(oxidant) Creation of O2 by photosynthesis
greatly enabled biological metabolism in
cells, since the free energy change is very
high Without O2, though, redox reactions still
occur, to exploit anaerobic environments
0.53 V
-0.05 V
-0.22 V -0.24 V
The redox reactions, giving negative free-energy
changes, are mediated biologically, mainly by
single-celled bacteria and archaea
13
standard hydrogen electrode E 0.0 V
Oxidation (anode) Zn ? Zn2 2e- Reduction
(cathode) 2H 2e- ? H2 (g) Overall reaction
2H(aq) Zn(s) ? Zn2(aq) H2(g)
E 0.76 V E E - 2.3RT/nF logQ DG -nFDE
14
(No Transcript)