Populations and Samples - PowerPoint PPT Presentation
Title:
Populations and Samples
Description:
Clinical trials conducted worldwide to study efficacy and safety of Cialis (Tadalafil) for ED ... In the Cialis trial, the baseline severity level was reported ... – PowerPoint PPT presentation
Number of Views:308
Avg rating:3.0/5.0
Title: Populations and Samples
1
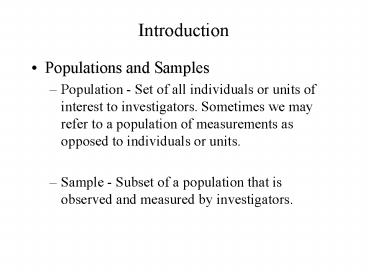
Introduction
- Populations and Samples
- Population - Set of all individuals or units of
interest to investigators. Sometimes we may refer
to a population of measurements as opposed to
individuals or units. - Sample - Subset of a population that is observed
and measured by investigators.
2
Quantitative and Qualitative Variables
- Quantitaive variables take on numeric values.
They can be further classified as - Continuous variables can take on values along an
interval (e.g. blood pressure, temperature) - Discrete variables can take on distinct values
with breaks (e.g. Womans parity, Number of
prior cardiac events) - Qualitative variables take on various categories.
They can be classified as - Nominal variables take on values with no inherent
ordering (e.g. Presence/Absence of parasite,
gender, race) - Ordinal variables take on categories that can be
ordered (e.g. Prognosis, Attitude toward a
proposal)
3
Dependent and Independent Variables
- Dependent variables are outcomes of interest to
investigators. Also referred to as Responses or
Endpoints - Independent variables are Factors that are often
hypothesized to effect the outcomes (levels of
dependent variables). Also referred to as
Predictor or Explanatory Variables - Research ??? Does I.V. ? D.V.
4
Example - Clinical Trials of Cialis
- Clinical trials conducted worldwide to study
efficacy and safety of Cialis (Tadalafil) for ED - Patients randomized to Placebo, 10mg, and 20mg
- Co-Primary outcomes
- Change from baseline in erectile dysfunction
domain if the International Index of Erectile
Dysfunction (Numeric) - Response to Were you able to insert your P
into your partners V? (Nominal Yes/No) - Response to Did your erection last long enough
for you to have succesful intercourse? (Nominal
Yes/No)
Source Carson, et al. (2004).
5
Example - Clinical Trials of Cialis
- Population All adult males suffering from
erectile dysfunction - Sample 2102 men with mild-to-severe ED in 11
randomized clinical trials - Dependent Variable(s) Co-primary outcomes listed
on previous slide - Independent Variable Cialis Dose (0, 10, 20 mg)
- Research Questions Does use of Cialis improve
erectile function?
6
Parameters and Statistics
- Parameters Numerical descriptive measures for
Populations - m - Mean (average) of a numeric variable
- s2 - Variance
- s - Standard deviation of a numeric variable
- CV - Coefficient of variation of a numeric
variable - p - Proportion of population with a nominal
characteristic
7
Parameters and Statistics
- Statistics Numerical descriptive measures for
Samples - Sample Mean (of a sample of size n)
- Sample Variance (s2) and standard deviation (s)
- Sample coefficient of variation (cv)
- Sample Proportion with a characteristic
8
Example - Carbonate of Bismuth
- Samples of Carbonate of Bismuth from a sample of
6 London manufacturing chemists - Measurements Quantity of Teroxide (Theoretically
should be 88.30 per 100 parts) - Measured levels 89, 88.5, 86.16, 87.66, 87.66, 86
Source Umney (1864)
9
Example - Clinical Trials of Cialis
- Among the 638 patients receiving placebo
(dose0), 198 responded Yes to Did your
erection last long enough for you to have
succesful intercourse? - Of 321 receiving 10mg dose, 186 replied Yes
- Of 1143 receiving 20mg dose, 777 replied Yes
Note that proportions are often reported as
percentages (number with characteristic per 100
exposed) or as rates per 10,000 such as mortality
rates for rare causes
10
Graphical Techniques
- Pictures are worth a bunch of words and computer
packages make graphing easy! - Histograms show the number or percent by category
or within ranges of values - Pie charts show proportionally the number or
percent by category or within ranges of values - Scatterplots plot a dependent variable on the
vertical axis versus an independent variable with
each subject being a point on the chart
11
Histogram of ED Severity Level
- In the Cialis trial, the baseline severity level
was reported for 2099 patients on an ordinal
scale 1Normal, 2Mild, 3Moderate, 4Severe
12
Pie Chart of ED Severity Level
13
Histogram of Disposition by Dose (Count)
Disposition 1Completed 2Adverse event 3Lack
of Efficacy 4Lost to follow-up 5Patient
Decision 6Protocol Violation 7Others
14
Scatterplot of Math Score vs LSD Level
- Response - Mean Math score for 7 subjects
- Predictor - Mean LSD Concentration
Conc Score 1.17 78.93 2.97 58.20 3.26 67.47 4.69
37.47 5.83 45.65 6.00 32.92 6.41 29.97
Source Wagner and Bing (1968)
15
Basic Probability
- Probability measures the likelihood or chances of
particular outcomes (or events) of random
experiment or observation - Let A and B be two events, with probabilities
P(A) P(B) - Intersection - Event that both A and B occur
(Notation AB) - Union - Event that either A and/or B occur
(Notation A?B) - Complement - Event that the event does not occur
(Notation A) - Probability Rules
P(A occurs Given B has occurred)
16
Example - High Cholesterol By Age and Sex
- WHO MONICA Survey of ?50000 Adults
- Proportions by Age, Gender, and Cholesterol
Male
Female
Source Gostynski, et al (2004)
17
Example - High Cholesterol By Age and Sex
- Probability a Randomly Selected Subject is Male
- Probability a Randomly Selected Subject is
over 40 years
- Probability Female given subject has High
Cholesterol
18
Independence
- Two events A and B are independent if
- P(AB) P(A) or, equivalently P(BA)
P(B) - Cholesterol Example
The occurrence of high cholesterol is not
independent of gender
19
Diagnostic Tests
- True state Disease Present (D) or Absent (D-)
based on a gold standard - Diagnostic test result Positive (T) or Negative
(T-) - Subjects can be classified in following table
(where a,b,c, and d are the number of subjects in
the 4 cells
20
Diagnostic Tests
- Sensitivity - The ability for the test to detect
that the disease is present P(T D) - Specificity - The ability for the test to detect
that the disease is absent P(T- D-) - Positive Predictive Value (PPV) - Proportion of
positive test results that actually have the
disease - Negative Predictive Value (NPV)- Proportion of
negative test results that do not have the
disease - Overall Accuracy - Proportion of subjects who are
correctly diagnosed
21
Diagnostic Tests
Assuming prevalence rates in test subjects is
same as in population
22
Example - Paracheck Test for Plasmodium
Falciparum (Pf)
- Goal Develop an inexpensive test for Pf in
asymptomatic children in remote parts of India - Gold Standard Microscopy
- Diagnostic Test Paracheck (0.65/test)
Source Singh, et al (2002)
23
Example - Paracheck Test for Plasmodium
Falciparum (Pf)
24
Basic Study Designs
- Studies can generally be classified as
observational or experimental - Observational - Subjects (or nature) select their
groups (levels of the independent variable) - Studies comparing ethnicities or sexes wrt drug
disposition - Studies of effects of smoking or other behaviors
- Studies comparing effects of patients on
different therapies - Experimental - Researchers assign subjects to
treatment groups - Clinical trials with patients being randomized to
active drug or placebo. Typically double-blind
(patient/assessor)
25
Observational Studies
- Case-Control -- Subjects are identified based on
presence/absence of the outcome of interest
(D.V.). It is then determined whether the subject
had been exposed to risk factor (I.V.).
Retrospective Studies. - Cohort -- Subjects are identified by risk factor
or treatment (I.V.) and followed over time to
observe outcome (D.V.). Prospective Studies. - Cross-sectional -- Subjects sampled at random
from population and levels of both I.V. and D.V.
are simultaneously observed. Many studies based
on large medical databases are cross-sectional
26
Example - Case-Control Study
- Purpose Study Risk Factors of Hepatitis-A in
Hispanic Children living in U.S. on Mexican
border (San Diego, CA) - Cases 132 Children with Hepatitis-A
- Controls 354 Children without Hepatitis-A
- Risk Factors
- Travel outside U.S. (67 of cases, 25 of cases)
- Eating food at taco stand/street vendor on travel
- Eating salad/lettuce on travel
Source Weinberg, et al (2004)
27
Example - Cohort Study
- Purpose Determine whether male adolescents who
develop schizophrenia were more likely to smoke
prior to onset - Subjects Israeli male military recruits, not
suffering major psychopathology who complete
smoking questionnaire - Cohorts 4052 smokers, 10196 non-smokers
- Follow-up/outcome 4-16 year follow-up for onset
of schizophrenia (20 smokers, 24 nonsmokers)
Source Weiser, et al (2004)
28
Example - Cross-Sectional Study
- Purpose - Investigate effect of high altitude on
maternal hemorrheology - Subjects - Pregnant and non-pregnant women at
high altitude and at sea level - Measurements - Blood/Plasma viscosities,
Hematocrit, total protein, Fibrinogen, Albumin - Selected Findings - Blood and Plasma viscosities
are higher in pregnant and non-pregnant women at
higher altitudes
Source Kametas, et al (2004)
29
Experimental Studies
- Randomized Clinical Trials - Studies where
investigators assign subjects at random to
treatments - Special Cases (more than one may apply)
- Parallel Groups - Each subject receives only one
treatment - Crossover - Each subject receives each trt (in
random order) - Placebo Controlled - One group receives only a
placebo - Double Blind - Subject nor assessor are aware of
which trt - Double Dummy - Subjects receive similar regimens
wrt appearance, when different drugs look
different - Intention-to-Treat - Analysis is based on all
subjects randomized, including those lost to
follow-up - Completed Protocol - Analysis based on only
subjects who completed study
30
Example - Randomized Clinical Trial
- Purpose - Three treatments for primary
dysmenorrhea in women - Subjects - 337 women (18-40) suffering
dysmenorrhea during past 3 consecutive menstrual
cycles - Treatments (Parallel Groups, double-blind,
double-dummy) - Group 1 1 tablet meloxicam 7.5mg o.a.d.
- 1 tablet placebo matching
meloxicam 15mg o.a.d. - 1 tablet placebo matching
mefenamic acid 500mg t.i.d. - Group 2 1 tablet meloxicam 15mg o.a.d.
- 1 tablet placebo matching
meloxicam 7.5mg o.a.d. - 1 tablet placebo matching
mefenamic acid 500mg t.i.d. - Group 3 1 tablet mefenamic acid 500mg t.i.d.
- 1 tablet placebo matching
meloxicam 7.5mg o.a.d. - 1 tablet placebo matching
meloxicam 15.0mg o.a.d. - Outcomes Ordinal global assessment of
safety/tolerability by patients and investigators
(Good, Satisfactory, Not satisfactory, Bad)
Source de Mello, et al (2004)