Gas Law Variables : - PowerPoint PPT Presentation
1 / 23
Title:
Gas Law Variables :
Description:
Boyle's Law : P and V of a gas at constant T are inversely proportional to each other. P V=k (k is a const.) Boyle's Law: P1 V1 = k = P2 V2. Inversely Proportional: ... – PowerPoint PPT presentation
Number of Views:474
Avg rating:3.0/5.0
Title: Gas Law Variables :
1
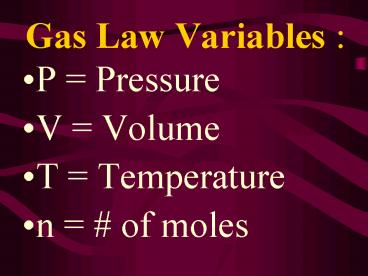
Gas Law Variables
- P Pressure
- V Volume
- T Temperature
- n of moles
2
Boyles Law
- P and V of a gas at constant T are inversely
proportional to each other. - PVk (k is a const.)
3
Boyles Law
- P1V1 k P2V2
4
Inversely Proportional
- as one increases, the other decreases
5
Problem
- The V of air in a bicycle pump 2 L and the P
1 atm. If the air is compressed to 0.5 L, what
is the new P?
4 atm
6
Problem
- P1 99.0 kPa
- V1 300 mL
- P2 188 kPa
- V2 ?
158 mL
7
STP
- Standard Temperature and Pressure
- T 0 C 273 K
- P 1 atmosphere
8
Charles Law
- V and T of a gas at constant P are directly
proportional to each other. - V ? T k
9
Charless Law
- V1
- T1
V2 T2
k
10
Gas Law Units
- Temperature units MUST use the Kelvin scale (K).
- P and V units can be any weve used, as long as
only one P and one V scale is used per problem.
11
Absolute Zero
12
Absolute Zero (0 K)
- T at which a gas would have NO volume and NO
motion - 0 K -273.15 C
13
Problem
- The volume of air in a balloon 2 L and its
temperature 280 K. After heating the air to
30?C, what does the new V?
2.16 L
14
Problem
- V1 3.00 L
- T1 80.0 C
- T2 30.0 C
- V2 ?
2.58 L
15
Gay-Lussac
- P and T of a gas at constant V are directly
proportional to each other. - P ? T k
16
Gay-Lussacs Law
- P1
- T1
P2 T2
k
17
Problem
- If the pressure of a gas is 1 atm at T 35ºC,
what is its new pressure at T 25ºC?
0.967 atm
18
Temperature Units
- MUST be in Kelvins
- ºC 273 K
19
Avogadros Principle
- Equal volumes of gases at the same T and P
contain equal numbers of particles. - V ? n k
20
Avogadros Principle
- V1
- n1
V2 n2
k
21
Gas Molar Volume
- The volume of 1 mole of gas.
- 22.4 L/mole at STP
22
- Direct Proportion (variation) y kx
- Straight line graph. Variables change in same
direction.
23
- Inverse Proportion (variation) y kx
- Curved-line graph. Variables changein opposite
directions.