Heat Energy - PowerPoint PPT Presentation
1 / 10
Title:
Heat Energy
Description:
... 10 kg stone is dropped into a 1000 g puddle of water from a height of 100 m. If ... to heat, by how much will the temperature of the water in the puddle raise? ... – PowerPoint PPT presentation
Number of Views:59
Avg rating:3.0/5.0
Title: Heat Energy
1
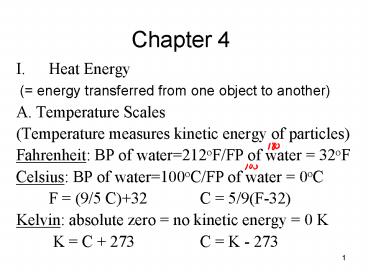
Chapter 4
- Heat Energy
- ( energy transferred from one object to
another) - A. Temperature Scales
- (Temperature measures kinetic energy of
particles) - Fahrenheit BP of water212oF/FP of water 32oF
- Celsius BP of water100oC/FP of water 0oC
- F (9/5 C)32 C 5/9(F-32)
- Kelvin absolute zero no kinetic energy 0 K
- K C 273 C K - 273
2
- Examples
- Normal body temperature is 98.6oF.
- What is this temperature in Celsius Kelvin?
- 2. Room temperature is about 23oC.
- What is this temperature in Fahrenheit?
3
- B. Methods of Heat Transfer
- 1. Conduction by direct contact,
- touches
directly - 2. Convection by currents or
- movement of fluids (liquids gases)
- (heat rises/cold sinks)
- 3. Radiation by electromagnetic waves,
- not through materials
- (examples x-rays, microwaves,
- ultraviolet waves, infrared waves)
4
- C. Calculating Heat Energy
- a. Heat to change Temperature m x Cp x ?T
- m mass of substance in kg
- Cp Heat Capacity
- ?T change in temperature (T2-T1)
- Usually measured by transferring heat
- to/from water, whose Cp 4.2 kJ/kgoC
5
- Examples
- 1. A 5 g piece of brass at a temperature of
100oCis placed into a cup containing 11.2 g of
water at a temperature of 22oC. The final
temperature of both the brass and the water is
25oC. What is the heat capacity of the brass?
6
- 2. A 10 kg stone is dropped into a 1000 g puddle
of water from a height of 100 m. If all of the
energy changes to heat, by how much will the
temperature of the water in the puddle raise?
7
- b. Heat to Change State
- Solids particles packed in rigid structures,
- attracted by opposite charges
- Liquids particles move around, but still remain
close - attracted by opposite charges
- Gases particles far apart, little or no
attraction - All energy used to pull molecules apart when
changing state, - No temperature change until done
8
- Heat of Fusion (Hf) energy gained/lost when
changing between solid liquid - Hf for water 335 kJ/kg
- Heat of Vaporization (Hv) energy gained/lost
when changing between gas liquid - Hv for water 2260 kJ/kg
- Break down problems into sections
- Heat to Melt Heat to change Temp Heat to
vaporize - Heat to condense Heat to change Temp Heat to
Freeze
9
- Example
- How much heat energy is needed to change
- 25 g of ice at 0oC to steam at 100oC?
10
- Physics Test Next Week
- You will be given the sheet of Physics Formulas
Tables - Study all material on Homework Assignments from
Weeks 1-4 - Test is mostly problems!