Review: Basic Thermodynamic Relationships
1 / 56
Title: Review: Basic Thermodynamic Relationships
1
Review Basic Thermodynamic Relationships
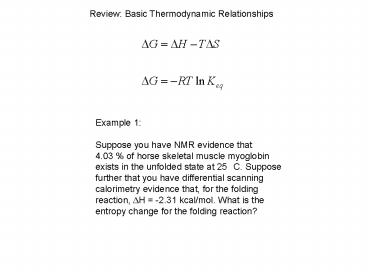
Example 1 Suppose you have NMR evidence
that 4.03 of horse skeletal muscle
myoglobin exists in the unfolded state at 25 C.
Suppose further that you have differential
scanning calorimetry evidence that, for the
folding reaction, DH -2.31 kcal/mol. What is
the entropy change for the folding reaction?
2
Example 1 Solution
1. Find Keq
2. Find DG
3. Find DS
Notice that you have been able to solve this
problem even though you did not know what kind
of NMR evidence was involved, or anything about
DSC.
3
Review A Simple Binding Equilibrium
In which P is free protein, L is free ligand, C
is protein-ligand complex, and KD is the
dissociation constant for the process. The
concentration of C as a function of total protein
and total ligand is given by
4
Review Binding of Oxygen to Myoglobin and
Hemoglobin
The binding of oxygen to myoglobin is described
by a simple single site binding isotherm binding
to hemoglobin is cooperative hemoglobin
exhibits allostery.
Figure from Voet and Voet, Biochemistry, 3rd
Edition.
5
Review of Allostery The Effect of pH on
the Hemoglobin Oxygen Interaction
More acidic pH reduces hemoglobins affinity for
oxygen more basic pH increases oxygen
affinity. (Which has important implications for
function)
Figure from Voet and Voet, Biochemistry, 3rd
Edition.
6
Review of Allostery The Effect of
2,3-bisphosphoglycerate on the Hemoglobin
Oxygen Interaction
Wikipedia
2,3-bisphosphoglycerate
The presence of 2,3-BPG reduces oxygen affinity
important implications for the function of
fetal hemoglobin.
7
Review The Michaelis-Menten Mechanism and
Equation
k1
kcat
E S
ES
E P
k-1
In which E is enzyme, S is substrate, ES is
enzyme-substrate intermediate, and P is product.
Making the steady state assumption for the
time-dependence of ES, defining
and in the initial rate regime, the rate of
product formation is given by the
Michealis-Menten Equation
8
Review Michealis-Menten Kinetics
Michealis-Menten kinetics exhibit a
characteristic shape, the rectangular hyperbola.
Vmax 12 s-1
½ Vmax At the substrate concentration equal to
KM, the reaction velocity is half maximal.
S KM 3
9
(No Transcript)
10
The Reactions of Glycolysis
Investment of 2 ATP Rxn 1 Hexokinase Rxn 3
Phosphofructo- kinase Production of
4 ATP Rxn 7 Phosphoglycerate
kinase Rxn 10 Pyruvate
kinase Production of 2 NADH Rxn 6
Glyceraldehyde-3-
phosphate-
dehydrogenase Overall Reaction glucose 2NAD
2ADP 2Pi ? 2pyruvate 2NADH 2ATP 2H2O
4H
11
The First Reaction The Phosphorylation of
Glucose Hexokinase Adds a Phosphate to Glucose
hexokinase
glucose ATP
glucose-6-phosphate ADP
Nucleophilic attack of the glucose-6-hydroxyl
group on the Mg2 - ATP complex of hexokinase.
First investment of ATP.
12
The Second Reaction The Isomerization of
Glucose-6-phosphate to Fructose-6-phosphate
glucose-6-phospahte
fructose-6-phospahte
13
The Second Reaction The Isomerization of
Glucose-6-phosphate to Fructose-6-phosphate
14
The Third Reaction Phosphofructokinase Adds a
Phosphate to Fructose-6-phosphate
fructose-6-phosphate
fructose-1,6-bisphosphate
Phosphofructokinase and hexokinase are
homologous enzymes, and their mechanisms are
similar
Second investment of ATP
15
The Fourth Reaction Aldolase Breaks the
6-Carbon Fructose-1,6-bisphosphate into the
3-Carbon Sugars Dihydroxyacetonephosphate and
Glyceraldehyde-3-phosphate
fructose-1,6-bisphosphate
dihydroxy-acetone phosphate
glyceraldehyde-3- phosphate
16
Aldolase Mechanism (I)
There are two general bases in the active site
K229 and D33.
Nucleophilic attack and hydrogen bonding in the
active site.
17
Aldolase Mechanism (II)
A covalent intermediate occurs.
Protonated Schiff base formation
18
Aldolase Mechanism (III)
(Schiff base lost.)
19
Aldolase Mechanism (IV)
The enamine intermediate undergoes
tautomerization and protonation and a Schiff
base reforms.
20
Aldolase Mechanism (V)
Regeneration of the free enzyme.
21
The Fifth Reaction Triose Phosphate
Isomerase Interconverts DHAP and GAP
22
The Fifth Reaction Triose Phosphate
Isomerase Interconverts DHAP and GAP
23
Detail of the TIM Active Site
A look at the coordination site for the phosphate
group. At left, the NH from the backbone of S211
and A212 on top, the NH from G171, and at right
that from G233. Note that phosphate binding
sites frequently involve back- bone atoms, and
glycine residues.
2-phosphoglycolate, His-95 and Glu-165 are shown
as sticks other active site residues as lines.
Note the positioning of the imidazole and acid
side chains in terms of the reaction mechanism,
as previously discussed.
24
The Sixth Reaction Glyceraldehyde Dehydrogenase
Oxidatively Adds Phosphate to Glyceraldehyde-3-pho
sphate
Figure Lehninger, 5th Edition
25
Elucidation of the Mechanism of GAPDH I
Sulfhydryl functions react readily with
iodoacetate (mechanism?) to yield (stable)
thio-ethers. Incubation of GAPDH with
iodoacetate followed by proteolysis yields the
carboxy-methyl adduct of cysteine, indicating the
presence of a free cysteine residue in the
protein. Because this also inactivates the
protein, the cysteine probably occurs at the
active site.
Figure VV 3rd Edition.
26
Elucidation of the Mechanism of GAPDH II
Use of a specifically 1-3H labeled GAP substrate
produces radioactive NADH, conclusively
identifying the source of the electron pair and
proton that are transferred to NAD in the
oxidation/reduction reaction. No radioactivity
occurs in solution the mechanism must involve
direct hydride transfer.
Figure VV 3rd Edition.
27
Elucidation of the Mechanism of GAPDH III
Acetyl phosphate is a product analog. Incubation
of acetyl phosphate in GAPDH solution containing
32P phosphate results in incorporation of
radio-phosphate into acetyl phosphate. This
suggests the mechanism involves an acyl-enzyme
intermediate.
Figure VV 3rd Edition.
28
Mechanism of GAPDH
Figure Lehninger, 5th Edition.
29
The Seventh Reaction Phosphoglycerate Kinase
Generates The First ATP From 1,3-bisphosphoglycer
ate
ADP
30
The Seventh Reaction Phosphoglycerate Kinase and
Hexokinase are Homologous Proteins and Exhibit
Similar Mechanisms.
PGK
Hexokinase
Direct transfer of a phosphate to ADP is
referred to as substrate level
phosphorylation as distinguished from
ATP production through oxidative phosphorylation.
Figures VV 3rd Edition.
31
The Eighth Reaction Phosphoglycerate Mutase
Catalyzes (Apparent) Phosphate Transfer from the
3 to the 2 Position
Why is this reaction important for glycolysis?
(Consider the following steps)
32
The Eighth Reaction Phosphoglycerate Mutase
Catalyzes (Apparent) Phosphate Transfer from the
3 to the 2 Position
Figure Lehninger, 5th Edition.
33
The Ninth Reaction Enolase Generates
Phospo-enol-pyruvate From 2-phosphoglycerate
34
Enolase I
K345 General Base K396, E211 General
Acids Mg2 ions stabilize charge
Figure VV, 3rd Edition.
35
Enolase II
The observation of proton exchange with solvent
indicates the following step is slow loss of
3-OH is not concerted
Figure VV, 3rd Edition.
36
Enolase III
Resolution to form phospho-enol-pyruvate
Figure VV, 3rd Edition.
37
The Tenth Reaction Pyruvate Kinase Resolves
Phospho-enol-pyruvate
Generation of ATP
Figure VV, 3rd Edition.
38
Regeneration of NAD, the Primary Oxidant of
Glycolysis.
Under anaerobic conditions muscle lactic
acid fermentation yeast alcohol
fermentation Under aerobic conditions electron
transport chain, oxidative phosphorylation
39
(No Transcript)
40
Lactic Acid Fermentation Involves the
Transformation of Pyruvate into Lactate
lactate dehydrogenase
NADH
pyruvate
lactate
In this reaction an equivalent of NADH is
reoxidized, and again becomes available for
glycolytic oxidations.
41
Structure of Lactate Dehydrogenase in Complex
with Linked Bi-substrate
Arg 171 forms a salt bridge with the lactate
(pyruvate) acid group. Arg 109 and His 195 make
H-bonds to the 2-alcohol (2-carbonyl). The redox
center of NADH is in close proximity to the
lactate (pyruvate).
42
Proposed Mechanism of the Lactate Dehydrogenase
Reaction
43
Overall Process and Energetics of Anaerobic
Glycolysis in Muscle
Glucose 2 ADP 2 Pi
2 lactate 2 ATP 2 H2O 2 H
DG - 196 kJ / mol
DG 61 kJ / mol
2 H2O 2 ATP
2 ADP 2 Pi
61 / 196 0.31 Lactic acid fermentation is
approximately 31 efficient (under standard
biochemical conditions) and is probably around 50
efficient under physiological conditions. The
remaining energy is lost as heat. (Although
lactic acid is reconverted into glucose in the
liver). What is the advantage? Lactic acid
fermentation proceeds about 100 X faster than
oxidative phosphorylation.
44
Anaerobic Fermentation in Yeast Produces CO2 and
Ethanol Ok. Thats pretty important stuff,
right? Its how you make beer, wine and
bread. Two Step Process
pyruvate decarboxylase
alcohol dehydrogenase
NADH
45
Pyruvate Decarboxylase Involves a Thiamine
Pyrophosphate Cofactor
Thiamine pyrophosphate (TPP). Acidity of the 2-H
on the thiazolium ring permits facile ylide
formation.
46
Experimental evidence from crystallography and
other basic biochemistry implies this mechanism
for ylide formation.
ylide formation requires deprotonation. The amino
function on pyridine ring involved in steric
clash not basic enough.
Glu 51 donates a proton to the pyridine ring,
driving imine formation, and deprotonation of
thialozium ring.
47
Proposed Mechanism for Pyruvate Decarboxylase
Reaction
48
Alcohol Dehydrogenase Interconverts Acetaldehyde
and Alcohol
alcohol dehydrogenase
NADH
Direction of the reaction depends on relative
concentrations of acetaldehyde and
alcohol. NADH is reoxidized in the formation of
the alcohol product and becomes available again
as an oxidant in glycolysis.
49
Mechanism of Alcohol Dehydrogenase in the
Direction of Aldehyde Reduction
50
Overall Process and Energetics of Anaerobic
Glycolysis in Muscle
DG - 235 kJ / mol
DG 61 kJ / mol
2 H2O 2 ATP
2 ADP 2 Pi
61 / 235 0.26 Alcohol fermentation is
approximately 26 efficient (under standard
biochemical conditions) and is probably around 50
efficient under physiological conditions. The
remaining energy is lost as heat. Aside from
beer, wine, and bread, what is the
advantage? Yeast may survive in solutions of up
to 12 ethanol. Most other microorganisms cant
tolerate ethanol above 5. Ethanol is a yeast
antibiotic.
51
The following slides are supplemental and may be
of use to you.
52
The Binding of Glucose to Hexokinase Induces
Conformational Change
Glucose free a cleft exists at the active site
of the enzyme.
Glucose bound the cleft closes around the
substrate.
Conformational changes are a general feature of
enzyme activity These occur on Substrate
binding Regulator binding
Figures VV 3rd Edition.
53
Aldol Cleavage
General base catalyzed mechanism of aldol
cleavage. Note that this is the reverse of the
aldol condensation mechanism by which two ketones
may be joined (a mechanism by which acetone
degrades)
54
The Fifth Reaction Triose Phosphate
Isomerase Interconverts DHAP and GAP
Ribbon diagram of TIM shown in complex with
2-phosphoglycolate (a transition-state
analog) the catalytic glutamic acid and
histidine residues are shown as sticks.
55
The Eighth Reaction Detail of the Active Site
of Phosphoglycerate Mutase
Figure VV, 3rd Edition.
56
Figure VV, 3rd Edition.