HMW
Title:
HMW
Description:
in 400 mL of a solution that is 0.100 M in ammonia. and diluting to a total volume of ... A chemist who works in the process laboratory of the Athabascha Alkali ... –
Number of Views:128
Avg rating:3.0/5.0
Title: HMW
1
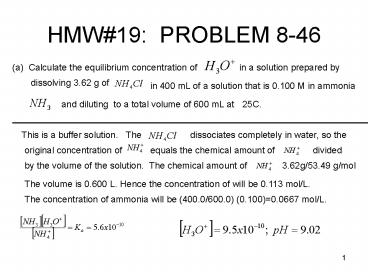
HMW19 PROBLEM 8-46
(a) Calculate the equilibrium concentration of
in a solution prepared by
dissolving 3.62 g of
in 400 mL of a solution that is 0.100 M in ammonia
and diluting to a total volume of 600 mL at
25C.
This is a buffer solution.
The
dissociates completely in water, so the
original concentration of
equals the chemical amount of
divided
by the volume of the solution. The chemical
amount of
3.62g/53.49 g/mol
The volume is 0.600 L. Hence the concentration of
will be 0.113 mol/L.
The concentration of ammonia will be
(400.0/600.0) (0.100)0.0667 mol/L.
2
- Suppose 0.0460 mol of HCl is added to the buffer
from part (a). Calculate - the pH of the solution that results at 25C.
0.0460 mol
0.600L(0.0667M)0.04mol
initial
final
0
0.0060mol
0.04 mol
0.0060mol/0.600L0.01M
pH-log(0.01)2
3
HMW19 PROBLEM 8-52
A total of 100.0 mL of a 0.3750 M solution of the
strong base
is tritrated at 25C with a 0.4540 M solution of
the strong acid
Compute the pH of the titration solution before
any acid is added, when the titration is 1.00 mL
short of the equivalence point, when the
titration is at the equivalence point, and when
the titration is 1.00 mL past the
equivalence point. (Caution Remember that each
mole of
gives 2 moles of
in solution.)
The strong base
dissociates essentially completely into ions as
it dissolves. Before the start of the titration
4
when the titration is 1.00 mL short of the
equivalence point
Reaching equivalence requires the addition of
75.00 mmol of
The volume of the
is
titrant that supplies 75.00 mmol of
The question concerns the pH when only 164.20 mL
of acid has been added. At this point
Unreacted
remains
At the equivalence point of a titration of a
strong base with a strong acid
pH7
5
At 1.00 mL past equivalence
The volume of
solution that has been added is 166.20 mL.
The
ion is now in excess
6
HMW19 PROBLEM 8-56
A total volume of 140.0 mL of 0.175 M aqueous
ammonia is titrated with 0.106 M HCl at 25 C.
Compute the pH before any HCl is added, when the
titration is at the half-equivalence point, when
the titration is at the equivalence point and
when the titration is 1.00 mL past the
equivalence point.
Before any titrant is added,
The solution is 0.175 M
Ammonia is a base in water.
.
initial
0.175
0
0
change
-x
x
x
0.175-x
final
x
x
7
when the titration is at the half-equivalence
point,
The half-equivalence in the titration of a weak
base with a strong acid the relationship
0
0
when the titration is at the equivalence point
(0.175)(140ml)24.5mmol so at equivalence you
need 24.5 mmol of H
How much volume of the acid will furnish these
many mmol?
8
When the titration is 1.00 mL past the
equivalence point,
You are going to have an excess of the strong
acid.
231.1 ml are needed of the acid to achieve
equivalence so a mL past is 232.1 ml
The difference in mmol will be (24.60-24.50)
0.1 mmol of acid
9
HMW19 PROBLEM 8-60
A chemist who works in the process laboratory of
the Athabascha Alkali Company makes frequent
analyses for ammonia recovered from the Solvay
process for making sodium carbonate. What is the
pH at the equivalence point if she titrates the
aqueous ammonia solution (approximately 0.10
mol/L) with a strong acid of comparable
concentration? Select an indicator that would be
suitable for the titration.
At the equivalence point in a titration of a 0.10
mol/L solution of the weak base ammonia by
hydrochloric acid of a similar concentration, the
chemist has a solution that is very close to
0.050 mol/L in ammonium ion.
initial
0.1 mol
0
0.1 mol
0
change
-0.1mol
-0.1 mol
0.1 mol
0.1 mol
Assume one L of each for a total volume of 2 L
so you will have 0.1/20.05 mol/L
10
(No Transcript)
11
HMW19 PROBLEM 8-63
- Examine the titration curve below (see pg. 394
of text) - Which of the following titrations could it
represent HCl by KOH, - RbOH by HBr, ammonia by nitric acid?
- (b) Choose a suitable indicator for signaling the
end-point of the titration. Justify - your answer.
- Suppose that the figure represents the titration
of 100.0 mL of NaOH by - a solution 0.065 M nitric acid.
Calculate the concentration of NaOH in - the original solution.
- The pH drops at the equivalence point from above
pH10 to below pH4.
This must be the titration of a base by added
acid. Such a drop in pH, moreover, could only
happen with the titration of RbOH by HBr
- The transition in pH occurs across the pH range
10-4. Many indicators would - work, including phenophthalein (transition
range ph10 to 8.2), cresol red - (pH8.8 to 7.0), bromothymol blue (pH7.6
to 6.0) and methyl red (6.0 to 4.8)
12
- The transition occurs after an equivalent amount
of acid has been added to - neutralize the base. The amount of acid
added was 0.30 Lx 0.065 M0.00195 mol - acid. This is equal to the mol of NaOH
present, so we get about 0.020 M for the - concentration of NaOH