TEAM PROJECTS Isoplethal Study - PowerPoint PPT Presentation
1 / 8
Title:
TEAM PROJECTS Isoplethal Study
Description:
Label all areas in the diagram. Make an isoplethal study in the system on cooling a melt of ... P. D. Garn and S. S. Flaschen, Anal. Chem., 29, 271-275 (1957) ... – PowerPoint PPT presentation
Number of Views:1112
Avg rating:5.0/5.0
Title: TEAM PROJECTS Isoplethal Study
1
TEAM PROJECTS (Isoplethal Study)
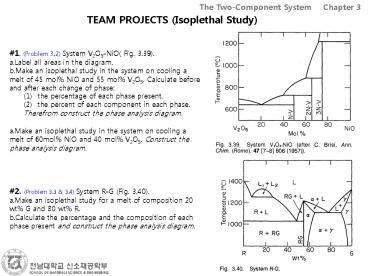
- 1. (Problem 3.2) System V2O5-NiO( Fig. 3.39).
- Label all areas in the diagram.
- Make an isoplethal study in the system on cooling
a melt of 45 mol NiO and 55 mol V2O5. Calculate
before and after each change of phase - Make an isoplethal study in the system on cooling
a melt of 60mol NiO and 40 mol V2O5. Construct
the phase analysis diagram.
- the percentage of each phase present.
- the percent of each component in each phase.
- Therefrom construct the phase analysis diagram.
- 2. (Problem 3.3 3.4) System R-G (Fig. 3.40).
- Make an isoplethal study for a melt of
composition 20 wt G and 80 wt R. - Calculate the percentage and the composition of
each phase present and construct the phase
analysis diagram.
2
TEAM PROJECTS (Phase Diagram Calculation)
3. (Problem 4.3) Construct the phase diagram for
the binary system CaO-MgO by calculating the
difference in free energy between the liquid and
solid phases at various temperatures. Find the
exact the eutectic point. Assume the system to be
a simple eutectic system. (Published data is
shown below). The melting point for CaO is 2630C
and its enthalpy of fusion is 12000 cal/mol. For
MgO, the melting point is 2825?C and the enthalpy
of fusion is 18500 cal/mol. Use two different
methods for ?G(GL-GS) estimation and compare the
results.
65.6
Phase Equilibria Diagrams Fig. 00229System
CaO-MgO. R. C. Doman, J. B. Barr, R. N. McNally,
and A. M. Alper, J. Am. Ceram. Soc., 46 7
313-316 (1963).
cf.) Fig.3.2
See Appendix 1 2 for wt - Mol conversion.
3
TEAM PROJECTS (Phase Diagram Calculation)
4. (Problem 4.5) Determine the slopes of the
liquidus lines in the system Li2O?B2O3 ?
Na2O?B2O3 from calculations of the freezing point
depression. Assume a simple eutectic system.
Compare your results with published data.
TE653
41.7
Phase Equilibria Diagrams Fig. 00429 System
Li2O? B2O3-Na2O?B2O3. H. S. Van Klooster, Z.
Anorg. Chem., 69 2 122-134 (1911).
4
TEAM PROJECTS (Phase Diagram Calculation)
5. (Problem 4.7) Determine the slopes of the
liquidus and solidus lines in the system KNbO3 ?
KTaO3 . Assume complete solid solution between
the end-members.
Phase Equilibria Diagrams Fig. EC-305 System
KNbO3-KTaO3. P. D. Garn and S. S. Flaschen,
Anal. Chem., 29, 271-275 (1957).
5
TEAM PROJECTS (Phase Diagram Calculation)
6. Determine the metastable immiscibility in
SiO2 ? Mullite system according to Risbud Pask.
liquidus temp /K
Fig. 06444System Al2O3-SiO2. . S. H. Risbud and
J. A. Pask, J. Am. Ceram. Soc., 60 9-10 418-424
(1977).
liquidus composition (mole fraction mullite)
6
TEAM PROJECTS 7,8,9
FeO-Al2O3-SiO2
1600?C
? refractory ?
7
7. Determine the liquid amount in Al2O3 ?SiO2
refractory reacted with 5 wt FeO at 1600?C.
8. Determine the liquid amount in mullite
refractory reacted with 5 wt FeO as a function
of temperature.
9. Determine the liquid amount in mullite
refractory as a function of reacted FeO amount at
at 1600?C.
8
(No Transcript)