Percent dissociation of weak acids - PowerPoint PPT Presentation
1 / 39
Title:
Percent dissociation of weak acids
Description:
It is harder to remove a proton from a negative ion than from a neutral molecule ... What are concentrations of species present in 0.020 M solution of carbonic acid? ... – PowerPoint PPT presentation
Number of Views:261
Avg rating:3.0/5.0
Title: Percent dissociation of weak acids
1
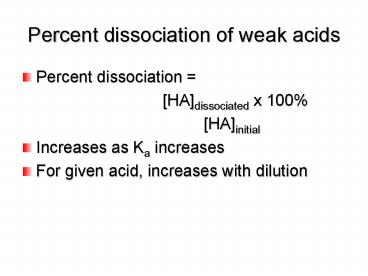
Percent dissociation of weak acids
- Percent dissociation
- HAdissociated x
100 -
HAinitial - Increases as Ka increases
- For given acid, increases with dilution
2
Dissociation increases with dilution
3
Polyprotic acids
- Dissociations are stepwise
- H2CO3 H2O H3O(aq) HCO3-(aq)
- HCO3-(aq) H2O H3O(aq) CO32-(aq)
4
Ka decreases with each step
- It is harder to remove a proton from a negative
ion than from a neutral molecule - Solution contains mixture of all species
- Strongest acid is the unionized molecule HnX
5
Use the same strategy described earlier
- What are concentrations of species present in
0.020 M solution of carbonic acid? - Step 1 initial species are H2CO3 and H2O
- Step 2 Ka1 Kw principal process is ionization
of H2CO3
6
ICE Age Two
7
Step 5 Obtain x from Ka
X ltlt0.02
8
Step 6 Big concentrations
- H3O HCO3- x 9.3 x 10-5 M
- H2CO3 0.020 x 0.020 M
9
Step 7 small concentrations
- Obtained from subsidiary equilibria
- HCO3-(aq) H2O H3O(aq) CO32-(aq)
- CO32- Ka2 5.6 x 10-11 M
- In general, A2- Ka2
Ignore H3O generated in second ionization
10
Step 7 contd.
- OH- from dissociation of water
11
Step 8 Calculate pH
- pH -log10H3O
- -log(9.3x10-5)
- 4.03
12
Weak base equilibria
- Treated in an analogous way to weak acids
13
Worked example
- Calculate pH and concentrations of species in
0.0012 M solution of codeine - Cod H2O CodH(aq) OH-(aq)
- Kb 1.6x10-6
- H2O H2O H3O(aq) OH-(aq)
- Kw 1.0 x 10-14
14
Principal reaction is protonation of codeine
15
Step 5 dissociation
X ltlt0.0012
16
Step 6 Big concentrations
- CodH OH- x 4.4x10-5 M
- Cod 0.0012 x 0.0012 M (x0.0012)
17
Step 7 small concentrations
- H3O from dissociation of water
18
Step 8 Calculate pH
- pH -log10H3O
- -log(2.3x10-10)
- 9.64
19
Relationship between Ka and Kb for conjugate
acid-base pair
- Acid
- HA H2O H3O(aq) A-(aq)
- Base
- A- H2O HA(aq) OH-(aq)
20
(No Transcript)
21
K for the overall reaction is product of Ks for
individual reactions
- In general
- Knet K1 x K2 x K3 x
- For conjugate acid-base pairs
- Ka x Kb Kw
22
Salts
- Products of acid-base neutralization
- HCl NaOH NaCl H2O
- HCl KOH KCl H2O
- HNO3 KOH KNO3 H2O
- 2HCl Ca(OH)2 CaCl2 2H2O
- HCN NaOH NaCN H2O
23
Unequally yoked
- Consequences for neutralized solutions of the
following combinations - Strong acid strong base
- NEUTRAL
- Strong acid weak base
- ACIDIC
- Weak acid strong base
- BASIC
24
Salts that yield neutral solutions
- Group 1A cations
- Group 2A cations (except Be2)
- Anions from strong monoprotic acids
- (Cl-, Br-, I-, NO3-, ClO4-)
25
Salts that yield acid solutions
- Salts of weak bases
- NH4(aq) H2O NH3 H3O(aq)
- Hydrated small polarizing metal cations
26
- What is pH of a 0.10 M solution of AlCl3
- Ka 1.4 x 10-5
- Using prior strategy
- Al(H2O)63, Cl- and H2O are initial species
- Al(H2O)63 is stronger acid than H2O
27
- pH -log(1.2 x 10-3) 2.92
X ltlt 1
28
Salts that yield basic solutions
- Salts of weak acid and strong base
- CN-(aq) H2O HCN(aq) OH-(aq)
- Calculation of pH follows same strategy as for
the salt of strong acid and weak base except use
expression for Kb
29
Weak acid and base what then?
- Competition between relative acid strength of
cation and base strength of anion - Consider (NH4)2CO3
- NH4(aq) H2O H3O(aq) NH3(aq) Ka
- CO32-(aq) H2O HCO3-(aq) OH-(aq) Kb
- If Ka gt Kb then pH lt 7
- If Ka lt Kb then pH gt 7
- If Ka Kb then pH 7
30
Summing up
31
Factors affecting acid strength
- How easily is the H X bond broken?
- the less easily dissociated, the weaker the acid.
- Bond strength
- Bond strength increases, acid strength decreases
- Polarity
- For a given bond strength, the more polar the
more easily dissociated closer to H
32
In a group, bond strength is the more important
factor
- Polarity is not a good predictor H-X polarity
decreases down the group as acid strength
increases - Bond strength decreases more strongly down the
group - Bond length increases sharply
33
Electronegativity more reliable indicator in a
period
- H-X polarity increases across period
- Acid strength increases across period
- Bond strength shows weaker trend
- Bond lengths vary little
34
Summing up
35
Oxoacids and strength where are the electrons
going
- How to weaken the OH bond and increase its
polarity? - Increase electronegativity of Y
- Increase oxidation number of Y (number of O atoms
around Y - Both serve to withdraw electrons from the H
making it more positive closer to H
36
Increasing electronegativity of Y
- In a homologous series, increasing
electronegativity of Y increases strength - Y draws charge away from the H atom, more easily
ionized - NOTE Opposite dependence to series HA, where A
F, Cl, Br, I
37
Increasing oxidation number (O atoms)
- More O atoms around Y, more acidic
- The O atoms draw charge away from the H atom,
more easily ionized - Also think in terms of relative stabilities of
the oxoanions formed in the process
38
Lewis acids no protons required
- Lewis acid an electron pair acceptor (BF3)
- Lewis base an electron pair donor (NH3)
BF3
NH3
DONOR
ACCEPTOR
39
Lewis definition casts the net further
- All Arrhenius acids are Brønsted acids
- All Brønsted acids are Lewis acids
- The converses are not true
- Examples
- Al3(acid) H2O (base)
- Cu2(acid) NH3 (base)
- SO3 (acid) H2O (base)