Recap of Lecture - PowerPoint PPT Presentation
1 / 1
Title:
Recap of Lecture
Description:
Strong acids dissociate completely to H and conjugate base. Know the strong acids. Weak acids dissociate partially. Use equilibrium calculation: Ka ... – PowerPoint PPT presentation
Number of Views:72
Avg rating:3.0/5.0
Title: Recap of Lecture
1
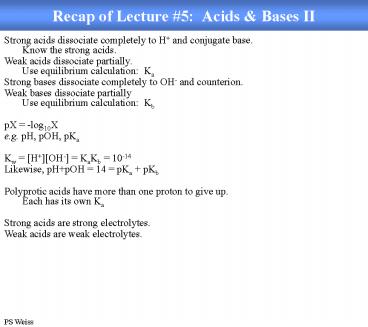
Recap of Lecture 5 Acids Bases II
Strong acids dissociate completely to H and
conjugate base.Know the strong acids. Weak acids
dissociate partially.Use equilibrium
calculation Ka Strong bases dissociate
completely to OH- and counterion. Weak bases
dissociate partiallyUse equilibrium calculation
Kb pX -log10X e.g. pH, pOH, pKa Kw
HOH- KaKb 10-14 Likewise, pHpOH 14
pKa pKb Polyprotic acids have more than one
proton to give up. Each has its own Ka Strong
acids are strong electrolytes. Weak acids are
weak electrolytes.