Reaction Equations
1 / 14
Title: Reaction Equations
1
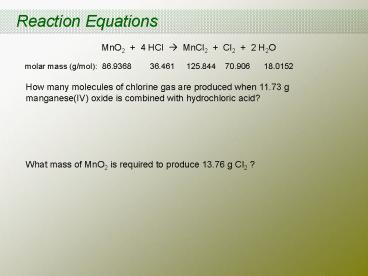
Reaction Equations
MnO2 4 HCl ? MnCl2 Cl2 2 H2O
molar mass (g/mol) 86.9368 36.461
125.844 70.906 18.0152
How many molecules of chlorine gas are produced
when 11.73 g manganese(IV) oxide is combined with
hydrochloric acid? What mass of MnO2 is
required to produce 13.76 g Cl2 ?
2
Percent Yield
During a chemical reaction, there may be reasons
that the recovered product is not exactly equal
to the amount predicted by the initial amount of
reactants. Theoretical yield is the amount of
product that should be recovered given some
amount of reactants. Actual yield is the amount
of product recovered from the reaction. Percent
yield actual yield x 100
theoretical yield AgNO3 KI ? AgI
KNO3 (molar masses AgNO3, AgI 169.88, 234.773
g/mol) 1.16 g of AgNO3 are combined with KI in
equal-molar ratio to produce 0.88 g of AgI. What
is the percent yield in the recovery of AgI?
(54.9 )
3
Classes of Chemical Reactions
Several types of reactions are known, we will
focus on three Precipitation reactions
processes in which an insoluble solid
(precipitate) forms when reactants are combined
in solution. Acid-base reactions processes in
which an acid reacts with a base especially
those that yield water plus an ionic compound (a
salt) Oxidation-reduction (redox) reactions
processes in which one or more electrons are
transferred between reactants.
4
Precipitation Reactions - Solubility
In a precipitation reaction, soluble reactants
are combined, which react and form an insoluble
compound. The insoluble compound precipitates
from solution as a solid. Many precipitation
reactions result when ionic compounds are
combined in water. The reactants dissociate in
water, and initially solvated ions are in
solution
CaCl2
Cl-
Ca2
Cl-
Cl-
Cl-
Ca2
5
Solubility of Ionic Compounds
There are numerous factors that influence the
solubility of ionic compounds. For reasons of
convenience, we will use some guidelines to
predict the solubility of an ionic compound in
water (of course, there are exceptions). General
Rules on Solubility Rule 1. A compound is
probably soluble if it contains one of the
following cations Group 1 cation Li, Na,
K, Rb, Cs Ammonium ion NH4 Rule 2. A
compound is probably soluble if it contains one
of the following anions Halide Cl-, Br-,
I-, except Ag, Hg22, Pb2, Hg2, Tl
Nitrate (NO3-), perchlorate (ClO4-), acetate
(CH3COO-), sulfate (SO42-) except Ba2,
Hg22, and Pb2 sulfates If a compound does not
contain at least one of the ions listed above,
then it is probably not soluble in water.
6
Precipitation Reactions
In a precipitation reaction, one combines two
aqueous solutions that contain ionic compounds.
Since these initial solutions contain water
soluble compounds, there are lots of dissolved
ions in solution. Ex solution 1 contains
ammonium sulfate solution 2 contains
barium chloride Solution 1 and solution 2 are
combined. Will a precipitate form? What is the
formula of the precipitate? If there is a
combination of ions within the combined solution
that can form a water insoluble ionic compound,
then precipitation of that insoluble compound
will occur. NH4 and Cl- ? soluble NH4 and
SO42- ? soluble Ba2 and Cl- ? soluble Ba2 and
SO42- ? INSOLUBLE!! BaSO4 will precipitate write
the reaction equation.
7
Precipitation Reactions
What is the identity of the yellow precipitate
that forms when lead nitrate (aq) and sodium
iodide (aq) are combined?
The yellow solid can be easily separated by
filtration.
8
Acid Base Reactions
Acid Base reactions in water Acid Base ?
Water (usually) Salt Strong acids A compound
that completely dissociates in water, yielding
the H ion. Ex HCl, HNO3, HBr, HClO4, HI,
H2SO4 Weak acids Generate H in water (by
partial dissociation). Ex CH3COOH, NH4, HSO4-,
H3PO4, and many more Strong bases A compound
that completely dissociates in water, yielding
the OH- ion. (or removes a proton from water)
Ex NaOH, KOH, Ca(OH)2, etc. Weak
bases Generate OH- in water through an
incomplete reaction with water. Ex NH3,
CH3COO-, etc. More details in Chapter 10!
9
Acid Base Reactions
When an acid and base are combined, they
neutralize each other. H in water OH- in
water ? H2O (neutral) What happens to the
other ions?? HX CatOH ? H2O CatOH X-
Cat ? CatX (a salt ? ionic
compound) Example HBr CsOH ? H2O
CsBr The formation of water molecules, which are
very stable, is a powerful driving force in
chemical reactions.
salt
10
Acid Base Reactions
Write and balance equations for the following
acid-base neutralization reactions CsOH
H2SO4 ? Ca(OH)2 CH3COOH ? NaHCO3
HBr ?
11
Aqueous Acids/Bases
H
H
H
basic O2- ? OH- ? H2O ? H3O acidic
oxide hydroxide water hydronium
When H (hydrogen ion) is available in water, it
protonates the water molecule. The result is
H3O (hydronium ion). In basic solutions,
deprotonated water molecules, or hydroxide ions
are more abundant than hydronium ions. Doubly
deprotonated water is the oxide ion. Pure water
is classified as neutral, being neither acidic
nor basic. (There is a small, but equal, amount
of hydronium and hydoxide)
12
Aqueous Acids/Bases
Acids
Bases
Strong Acid
Weak Acid
Weak Base
Strong Base
Substances that partially protonate water
Substances that partially deprotonate water
Substances that completely deprotonate water (or
are deprotonated water, ie hydroxide ion)
Substances that completely protonate water
HI CH3COOH CH3COO- NaOH H2SO4 NH4
NH3 NH2- HBr H2CO3 CO32- H- HCl H3O
H2O O2-
more H3O in solution
more OH- in solution
(neutral)
13
Reaction Equations
14
Reaction Equations