I' Diatomic Molecules - PowerPoint PPT Presentation
1 / 5
Title:
I' Diatomic Molecules
Description:
I. Diatomic Molecules. 1. ... I. Diatomic Molecules. 2. Symmetry and designation of electronic states of ... I. Diatomic Molecules. 3. Born-Oppenheimer ... – PowerPoint PPT presentation
Number of Views:108
Avg rating:3.0/5.0
Title: I' Diatomic Molecules
1
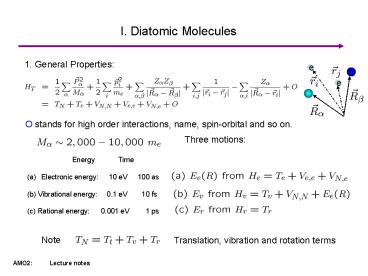
I. Diatomic Molecules
1. General Properties
O stands for high order interactions, name,
spin-orbital and so on.
Three motions
- Energy Time
- Electronic energy 10 eV 100 as
- (b) Vibrational energy 0.1 eV 10 fs
- (c) Rational energy 0.001 eV 1 ps
Note
Translation, vibration and rotation terms
2
I. Diatomic Molecules
Born-Oppenheimer approximation
With
Note that BO approximation is valid for most
molecules, not only the diatomic molecules
3
I. Diatomic Molecules
2. Symmetry and designation of electronic states
of diatomic molecules
If we use a cylindrical coordinate and make
transformation
(a)
(b) Total electron spin S,
(c) Reflection on a plane contains the molecular
axis,
(d) Reflection at the origin for homonuclear
molecules,
For homonculear molecules
4
I. Diatomic Molecules
Depend on the system
Symmetry
(a)
(b)
Note for ? ? 0, the energies of ? states are
degenerate.
(c)
(d)
notation
5
I. Diatomic Molecules
3. Born-Oppenheimer approximation
The exact total wave can be written as
with
Born-Oppenheimer approximation requires that
with
Thus, we separate the electron and nuclear
motions.