WaveParticle Duality - PowerPoint PPT Presentation
Title: WaveParticle Duality
1
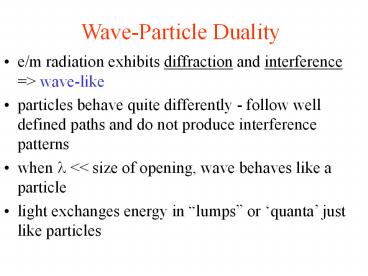
Wave-Particle Duality
- e/m radiation exhibits diffraction and
interference gt wave-like - particles behave quite differently - follow well
defined paths and do not produce interference
patterns - when ? ltlt size of opening, wave behaves like a
particle - light exchanges energy in lumps or quanta
just like particles
2
Water waves flare out when passing through
opening of width a
a
?
3
Wave-Particle Duality
- 1900 sound, light, e/m radiation were waves
- electrons, protons, atoms were
particles - 1930 quantum mechanics provided a new
interpretation - light behaves as a particle photoelectric
Compton effect - Ehf hc/?
ph/? - particles behave as waves electron diffraction
- gt localized packets of energy gt particle-like
- f, ? wave-particle duality E,p
light
electron
http//www.colorado.edu/physics/2000
4
Double Slit Experiment with electrons (1989)
5
Modern Physics
Large objects small speeds Newtonian Physics
F ma
Large objects large speeds relativistic
mechanics F dp/dt
size
Atomic scales small speeds Quantum
Mechanics Schrödinger Equation
Atomic particles Large speeds relativistic
quantum mechanics Dirac Equation
speed
6
Electromagnetic Waves
- Maxwell(1860) showed that light is a travelling
wave of electric and magnetic fields - E Em sin (kx-?t)
- B Bm sin (kx-?t)
- v ?/k c 3 x 10 8 m/s
- the speed is the same in all reference frames
- v c/n in material media ( n1 for vacuum)
7
Transverse Wave E and B are both ? to v and
E ? B
8
Light
- Light is a wave c?f
- gt exhibits interference and diffraction
- gt oscillating electric and magnetic fields are
solutions of Maxwells equations - gt Maxwells equations predict a continuous range
of ?s from ?-rays to long radio waves - electromagnetic spectrum
9
Electromagnetic Spectrum
Power ? ?2
10
Sensitivity of eye to various ?
11
Radiation
- heated objects glow if the temperature
is high enough - gtembers in a fire, stove element
- gt bar of steel heated to 12000 K glows in
deep red colour - thermal radiation
- charges in material vibrate in SHM(accelerate)
and produce e/m radiation - also occurs at lower T but ? is longer gt
infra-red and not visible
12
R(?,T)
14500K
Classical prediction for 14500 K
Cannot explain the peak
Watts m-2s-1
12500K
10000K
As T decreases, ? of peak increases
?
Partially explained by Planck 1900
13
Modern Physics
- 1905 Einstein proposed
- when an atom emits or absorbs light, energy
- is not transferred in a smooth continuous fashion
but rather in discrete packets or lumps of
energy - photons have energy Ehf
Frequency c?f
Plancks constant h6.63x10-34 J.s
14
Modern Physics
- h plays a similar role to c in relativity
- if c ? ? then no relativity! v/c ltlt1
always gt signals transmitted instantaneously - if h ? 0 then no quantum mechanics gt no
stable atoms!
15
Example
- Consider a 100W sodium vapour lamp with ?
590 nm - what is the energy of a single photon?
- Ehf hc/? (6.63x10-34
J.s)(3x108 m/s)/590x10-9 m) 3.37x10-19 J - Power dE/dt number of
photons/sec x 3.37x10-19 J 100 W - number of photons/sec 3 x 1020
16
Example
- The amount of sunlight hitting the earth is about
1000 W/m2 and ? 500 nm - photons/sec/m2 2.5x 1021
- we do not see the grainy character of the energy
distribution gt appears continuous - photoelectric effect (lab 4)
- if we shine a beam of light of short enough ?
onto a clean metal surface, the light will knock
electrons out of the metal surface