Chapter 7: Alkenes: Reactions and Synthesis - PowerPoint PPT Presentation
1 / 26
Title:
Chapter 7: Alkenes: Reactions and Synthesis
Description:
Nu-- E Nu. electrophile. nucleophile. E Nu-- 7-3. 1. Addition ... Pt - metal catalyst which is solid. Raney Nickel (finely divided) is also used. Key Features ... – PowerPoint PPT presentation
Number of Views:243
Avg rating:3.0/5.0
Title: Chapter 7: Alkenes: Reactions and Synthesis
1
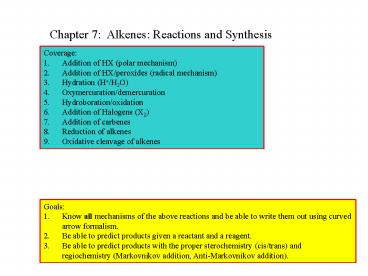
Chapter 7 Alkenes Reactions and Synthesis
- Coverage
- Addition of HX (polar mechanism)
- Addition of HX/peroxides (radical mechanism)
- Hydration (H/H2O)
- Oxymercuration/demercuration
- Hydroboration/oxidation
- 6. Addition of Halogens (X2)
- 7. Addition of carbenes
- 8. Reduction of alkenes
- Oxidative cleavage of alkenes
- Goals
- Know all mechanisms of the above reactions and be
able to write them out using curved arrow
formalism. - Be able to predict products given a reactant and
a reagent. - Be able to predict products with the proper
sterochemistry (cis/trans) and regiochemistry
(Markovnikov addition, Anti-Markovnikov addition).
2
The most common reaction of alkenes is
electrophilic addition.
E
Nu--
loosely held ? electrons
E
Carbocation intermediate
electrophile nucleophile
E
E
Nu
Nu--
3
1. Addition of Hydrogen Halides
4
What happens when an asymmetric alkene reacts
with HBr?
a
30
b
10
Which pathway is favored. Answer Pathway a
is favored since a more stable carbocation is
formed.
Markovnikovs Rule In electrophilic addition to
an alkene, the more stable carbocation forms in
preference to the least stable carbocation.
Restated, the nucleophile (X-) adds to the most
substituted carbon atom.
5
100 0
Mechanism
30 carbocation
Carbocation Rearrangements may occur.
6
2. Free Radical Addition of HBr (not HCl or HI)
Peroxides
0 100
Anti-Markovnikov Product
Mechanism Free radical chain reaction
- Initiation
- R-O-O-R 2 R-O.
- R-O. HBr R-O-H Br.
warm
7
Propagation
a. b.
.
30 radical stable
Br.
.
What is the alternative reaction in step (a)?
Answer It is possible to form a 20 radical.
However, since it is less stable,
it does not form!
8
3. Addition of H2O (Hydration)
H
Markovnikovs Rule is followed, that is, the
nucleophile (H2O) adds to the most substituted
carbon atom.
9
Mechanism, illustrated with 2-methylpropene
Protonation
Nucleophilic attack
Deprotonation
H3O
Are rearrangements possible with this reaction?
Answer Yes, as there are carbocation
intermediates.
10
- 4. Oxymercuration Demercuration
- Hydration with a mercury intermediate to form
an alcohol - Follows Markovnikovs rule more substituted
alcohol forms. - Advantage No carbocation intermediates, so
no rearrangements
Oxymercuration
Demercuration
Mercury(II) acetate
NaBH4 sodium borohydride
11
a. Oxymercuration
Cyclic Intermediate
1. 2.
b. Demercuration
Hg0 -OAc
12
Markovnikovs Rule is followed
Why? There is no carbocation intermediate here
The positive charge on the mercury is shared by
the carbon atoms. The 3o carbon bears a larger
positive charge, so H2O attacks the 3o carbons
leading to Markovnikov product.
?
?
Larger positive charge on 3ocarbon.
13
What about cycloalkenes?
In the above mercury above intermediate, the OH
and Hg are trans to each\ other. This type of
addition is termed anti addition, as opposed to
syn addition, where the two groups end up cis to
each other
14
Tamoxifen (Tamoxifen Citrate)
It is the trans-isomer of a triphenylethylene
derivative and is active in the treatment of
breast cancer, an oestrogen antagonist. Its cis
isomer has no clinical uses and is not an
oestrogen antagonist. Tamoxifen works against
the effects of estrogen on these cells (an
anti-estrogen) slows the growth of cancer
cells and prevents original breast cancer from
returning.
15
- 5. Hydroboration/oxidation
- Hydration of alkene to alcohol
- Anti-Markovikov addition
- Syn Addition
Boron Chemist Extraordinaire Herbert C. Brown,
Nobel Prize, 1979
a. Hydroboration
Alkyl borane Trialkyl
borane
Mechanism
?
empty p orbital
BH3-alkene complex
16
The boron and hydrogen add simultaneously to the
same side of the double bond termed syn
addition.
What about an asymmetric alkene?
The boron adds mostly to the least hindered
carbon.
Boron adds here
This is Anti-Markovnikov addition
17
b. Oxidation of trialkylborane
H2O2, OH-, H2O
3
Reaction of Cycloalkenes
Syn addition
trans product (no cis)
18
6. Catalytic Hydrogenation
H2, Pt
H2 gaseous at high pressure Pt - metal
catalyst which is solid. Raney Nickel
(finely divided) is also used.
- Key Features
- Heterogeneous catalysis gas, liquid and solid
phases are all present - Reaction occurs at surface of metal catalyst.
- High surface area of metal is desired.
- Syn addition takes place due to reaction at
surface.
19
7. Addition of Carbenes
dibromocarbene methylene
Carbenes are electron-deficient and react with
double bonds.
cyclopropane ring
20
Generation of Carbenes
a. Heating of diazomethane, CH2N2
Resonance forms
b. Haloform and Base
Dichlorocarbene
CHBr3 also works!
21
c. Simmons-Smith Reagent
Acts like CH2
Examples
22
8. Addition of Halogens, Br2 and Cl2
- Intermediate cyclic bromonium ion
- Anti addition results in trans product.
Bromonium ion intermediate
Dibromide
anti addition trans product
23
Variation Halohydrin formation
Add some water into the reaction. What happens?
bromohydrin
24
9. Oxidation of Alkenes
a. Syn Hydroxylation to diols
diol
H2O2
- Both oxygens are delivered to same side of
double bond simultaneously - therefore syn addition.
- Mechanism is similar using KMnO4
25
cis diol
b. Ozonolysis - cleaves the double bond
ketone aldehyde
Mechanism
..
..
..
..
..
..
..
..
..
..
ozonide
molozonide
..
26
..
(CH3)2S
..
..
dimethylsulfide
ozonide