Methane Conversion to Methanol by Platinum Catalysts - PowerPoint PPT Presentation
1 / 21
Title:
Methane Conversion to Methanol by Platinum Catalysts
Description:
All of these catalyst have been studied to see which ... Industry applications to produce natural gas to liquid products in milder conditions (T 300oc) ... – PowerPoint PPT presentation
Number of Views:437
Avg rating:3.0/5.0
Title: Methane Conversion to Methanol by Platinum Catalysts
1
Methane Conversion to Methanol by Platinum
Catalysts
- By Sean W. Hanley
2
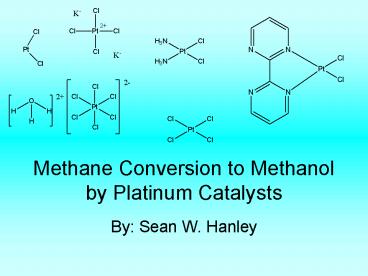
Platinum Catalysts
(NH3)2PtCl2
K2PtCl4
PtCl2
Platinum (II) Chloride
H2PtCl6
(bpyrm)PtCl2
All of these catalyst have been studied to see
which catalyst has the best percent yield of
methanol with the least amount of catalytic
death. (bpym)PtCl2 with oleum has been recently
found to have 72 yield. This catalyst will be
the major catalyst looked at. This reaction has
the most hope in industry but also a crucial
problem arises. Past catalysts have had 40 yield
using mercury which is not environmentally
friendly.
3
Overall Reactions
Overall Reaction
220C
Hydrolysis Reaction
Application Reaction
4
Catalytic Interaction
Platinum (II)
Platinum (IV)
Catalytic interaction mechanism to form methanol
Activating step of methane
5
Catalytic Interaction cont.
Suggested mechanism for the oxidation step
6
Catalytic Interaction cont.
Purposed mechanism for functionalization step
Final product then undergoes hydrolysis and turns
into methanol
7
Overall Proposed Mechanism
8
Different Solvent Interactions
- H2SO4 Sulfuric Acid
- Concentrated Sulfuric Acid
- Dilute Sulfuric Acid
- HF/SbF5 Causes Fluorination rather then
oxidation of methane - Methanol and H2O Interacts with catalyst- causing
catalytic death
9
Using Different Catalysts
- (bpym)PtCl2 soluble and good interaction with
sulfuric acid - PtCl2 and PtO2 insoluble in H2O or other organic
solvents - PtCl4, K2PtCl4, and H2PtCl6 soluble but not
compatible with sulfuric acid - This leads us to believe only (bpym)PtCl2 is the
only catalyst that works - Yet Ionic Liquids allow these other catalysts to
dissolve readily upon heating
10
Ionic Liquids (IL)
11
Ionic Liquids Interactions
12
Ionic Liquids Interactions Cont.
- Better percent yield (Concentration) from the
Ionic Liquids then with (bpym)PtCl2 by itself - Ionic liquids are more water-tolerant
- Green Chemistry due to low volatility and good
thermal stability
13
Applications
- Industry applications to produce natural gas to
liquid products in milder conditions (T lt 300oc) - Use in hydrogen cars to produce a hydrogen
yielding reaction from methanol - Safer fuels
- Environmentally
- friendly reaction
14
Batteries
15
Cell Phones
- You need only 2ml of fuel to ensure power for 20
hours operation of an MP3 audio player. Toshiba
is actually running field tests with mobile
phones at this moment.
16
Problems Facing Industry
- (bpym)PtCl2 is extremely sensitive to small
amounts of water present in the reaction - Methanol is very hard to extract from the
sulfuric acid/ water solution - There is also severe inhibition from the sulfuric
acid/ water solution- causing catalytic death
17
Future Experiments
- Differing acids may lead to a more accessible way
of extracting Methanol as a product - Using Ionic Liquids to create a higher yield in
less concentrated sulfuric acid - Different Platinum catalysts that are more
water-tolerant
18
Highlights
- (bpym)PtCl2 is a very good catalyst at producing
methanol approximately 70 yield - The problems are that methanol is very hard to
extract from the sulfuric acid/ water solution - HF/SbF5 is not a reasonable system to convert
methane to methanol - Ionic liquids increase yield and are more
water-tolerant - Realize how this catalyst could be used to
further Hydrogen Car applications
19
References
- Cheng, J. Li, Z. Haught. M. Tang. Y. Chem.
Commun. 2006, 4617 - Kua, J. Xu X. Perenia, R. A. Goddard, W. A.,
III. Organometallics 2002, 21, 511. - Kua, J. Goddard III, W. J. Am. Chem. Soc. 1999,
121, 10928 - Seidel, S. Seppelt, K. Inorg. Chem. 2003, 42,
3846 - http//www.fuelcells.org/
20
Questions???
21
- Cool Website Link to See Fuel Cells
- http//www.utcpower.com/fs/com/Videos/UTCPower.wmv