Unlike inorganic catalysts, enzymes are specific - PowerPoint PPT Presentation
1 / 62
Title:
Unlike inorganic catalysts, enzymes are specific
Description:
... number of moles of substrate converted to product per mole of enzyme per ... 10-4M is mediocre. 10-3M is fairly poor. So Km and k3 quantitatively characterize ... – PowerPoint PPT presentation
Number of Views:94
Avg rating:3.0/5.0
Title: Unlike inorganic catalysts, enzymes are specific
1
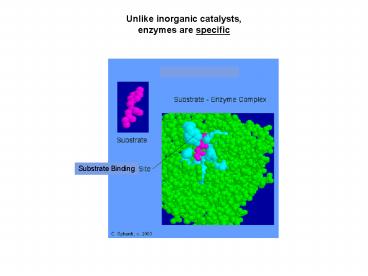
Unlike inorganic catalysts, enzymes are specific
2
Unlike inorganic catalysts, enzymes are specific
- succinic
dehydrogenase - HOOC-HCCH-COOH lt-------------------------------gt
HOOC-CH2-CH2-COOH
2H - fumaric acid
succinic acid - NOT a substrate for the enzyme
- 1-hydroxy-butenoate
HO-CHCH-COOH - (simple OH instead of one of
the carboxyl's) - Maleic acid
-
-
maleic
acid - Platinum will work with all of these,
indiscriminantly
3
- Enzymes work as catalysts for two reasons
- They bind the substrates putting them in close
proximity. - They participate in the reaction, weakening the
covalent bonds - of a substrate by its interaction with the
enzymes amino acid residue side groups (e.g.,
by stretching).
Movie http//www.columbia.edu/cu/biology/courses/
c2005/images/dhfr_movie2.gif
4
Chemical kinetics
- Substrate ? Product
- (reactants in enzyme catalyzed reactions are
called substrates) - S ? P
- Velocity V ?P/ ? t
- So V also -?S/ ?t (disappearance)
- From the laws of mass action
- ?P/ ?t - ?S/ ?t k1S k2P
- For the INITIAL reaction, P is small and can be
neglected - ?P/ ?t - ?S/ ?t k1S
- So the INITIAL velocity Vo k1S
5
Vo ?P/ ? t
P vs. t Slope Vo
6
Effect of different initial substrate
concentrations
0.6
S4
S3
0.4
P
S2
0.2
S1
0.0
t
7
Vo the slope in each case
Effect of different initial substrate
concentrations
0.6
S4
S3
0.4
P
S2
0.2
S1
0.0
t
Vo k1S
Slope k1
Considering Vo as a function of S (which wil be
our usual useful consideration)
8
Now, with an enzyme
We can ignore the rate of the non-catalyzed
reaction
9
Enzyme kinetics (as opposed to simple chemical
kinetics)
Vo independent of S
Vo proportional to S
Can we understand this curve?
10
Michaelis and Menten mechanism for the action of
enzymes (1913)
11
Michaelis-Menten mechanism
X
- Assumption 1. E S lt--gt ES this is how enzymes
work, via a complex - Assumption 2. Reaction 4 is negligible, when
considering INITIAL velocities (Vo, not V). - Assumption 3. The ES complex is in a
STEADY-STATE, with its concentration unchanged
with time during this period of initial rates. - (Steady state is not an equilibrium condition, it
means that a compound is being added at the same
rate as it is being lost, so that its
concentration remains constant.)
12
(No Transcript)
13
E S
ES
E P
14
Michaelis-Menten Equation(s)
See handout at your leisure for the derivation
(algebra, not complicated, neat)
k3EoS
Vo
(k2k3)/k1 S
If we let Km (k2k3)/k1, just gathering 3
constants into one, then
15
All the ks are constants for a particular enzyme
Rate is proportional to the amount of enzyme
At high S (compared to Km), Rate is constant Vo
k3Eo
At low S (compared to Km), rate is proportional
to S Vo k3EoS/Km
16
At high S, Vo here k3Eo, Vmax
So the Michaelis-Menten equation can be written
k3 Eo S
Vmax S
Simplest form
Vo
Vo
Km S
Km S
17
- Now, Vmax k3Eo
- So k3 Vmax/Eo
- the maximum (dP/dt)/Eo, the maximum
(-dS/dt)/Eo - k3 the TURNOVER NUMBER
- the maximum number of moles of substrate
converted to product per mole of enzyme per
second - max. no. of molecules of substrate converted to
product per molecule of enzyme per second - Turnover number then is a measure of the
enzyme's catalytic power.
18
Some turnover numbers (per second)
- Succinic dehydrogenase 19 (below average)
- Most enzymes 100 -1000
- The winner
- Carbonic anhydrase (CO2 H20 H2CO3)
- 600,000
- Thats 600,000 molecules of substrate, per
molecule of enzyme, per second. - Picture it!
- You cant.
19
Km ?
Vmax/2 is achieved at a S that turns out to be
numerically equal to Km
So Km is numerically equal to the concentration
of substrate required to drive the reaction at ½
the maximal velocity Try it Set Vo ½ Vmax and
solve for S.
20
Another view of Km
Consider the reverse of this reaction (the
DISsociation of the ES complex)
The equilibrium constant for this dissociation
reaction is
Kd ES / ES k2/k1
(Its the forward rate constant divided by the
backward rate constant. See the Web lecture if
you want to see this relationship derived)
21
Consider in reverse
Kd k2/k1
Km (k2k3)/k1 (by definition)
IF k3 ltlt k2, then Km k2/k1 But k2/k1 Kd
(from last graphic) so Km Kd for the
dissociation reaction (i.e. the equilibrium
constant)
(and 1/Km the association constant)
So the lower the Km, the more poorly it
dissociates. That is, the more TIGHTLY it is held
by the enzyme
And the greater the Km, the more readily the
substrate dissociates, so the enzyme is binding
it poorly
22
Km ranges
- 10-6M is good
- 10-4M is mediocre
- 10-3M is fairly poor
So Km and k3 quantitatively characterize how an
enzyme does the job as a catalyst
k3, how good an enzyme is in facitiating the
chemical change (given that the substrate is
bound) Km, how well the enzyme can bind the
substrate in the first place
23
Enzyme inhibition competitive, non-competitive,
and allosteric
A competitive inhibitor resembles the substrate
24
Competitive inhibitor can be swamped out at high
substrate concentrations
Handout 5-3b
25
-
Vo
Substrate concentration
Inhibitor looks like the substrate and like the
substrate binds to the substrate binding site
26
Biosynthetic pathway to cholesterol
27
Zocor(simvastatin)
28
½ Vmax w/o inhibitor
½ Vmax withyet more inhibitor
Km remains unchanged. Vmax decreases.
29
Substrate
Non-competitive inhibitor
Example Hg ions (mercury) binding to SH groups
in the active site
30
Non-competitive inhibitor example Substrate still
binds OK But an essential participant in the
reaction is blocked (here, by mercury binding a
cysteine sulfhydryl)
31
(No Transcript)
32
Allosteric inhibition
Inhibitor binding site
Active
Inactive
Active
allosteric inhibitor
substrate
Allosteric inhibitor binds to a different site
than the substrate, so it need bear no
resemblance to the substrate
The apparent Km OR the apparent Vmax or both may
be affected. The affects on the Vo vs. S curve
are more complex and ignored here
33
Allosteric inhibitors are used by the cell for
feedback inhibition of metabolic pathways
Feedback inhibition of enzyme activity, or End
product inhibition
First committed step is usually inhibited
34
Thr deaminaseglucose ...... --gt
--gt threonine -----------------gt
alpha-ketobutyric acid
protein
A
Substrate
B
C
isoleucine (and no other aa)
protein
Allosteric inhibitor Also here Feedback
inhibitor (is dissimilar from substrate)
35
Rich medium provide glucose all 20 amino
acids and all vitamins, etc.
60 minutes, in a minimal medium
20 minutes !, in a rich medium
36
Direction of reactions in metabolism
37
Free
determines the direction of a chemical reagion
38
For the model reaction A B C D,
written in the left-to-right direction
indicated
- Consider the quantity called the change in free
energy associated with a chemical reaction, or
? G - Such that
- IF ? G IS lt0THEN A AND B WILL TEND TO PRODUCE C
AND D(i.e., tends to go to the right). - IF ? G IS gt0THEN C AND D WILL TEND TO PRODUCE A
AND B.(i.e., tends to go to the left) - IF ? G IS 0THEN THE REACTION WILL BE AT
EQUILIBRIUM - NOT TENDING TO GO IN EITHER DIRECTION IN A NET
WAY.
39
- ?G ? Go RTln(CD/AB)
- where A, B, C and D are the concentrations of the
reactants and the products AT THE MOMENT BEING
CONSIDERED.(i.e., these A, B, C, Ds here are
not the equilibrium concentrations) - R UNIVERSAL GAS CONSTANT 1.98 CAL / DEG K
MOLE (R 2) - T ABSOLUTE TEMP ( oK ) 0oC 273oK Room temp
25o C 298o K (T 300) - ln NATURAL LOG
- ? Go a CONSTANT a quantity related to the
INTRINSIC properties of A, B, C, and D
40
- Also abbreviated form? G ? Go RTlnQ (Q for
quotient) - Where Q (CD/AB)
Qualitative term
Quantitative term
41
? Go
- STANDARD FREE ENERGY CHANGE of a reaction.
- If all the reactants and all the products
are present at 1 unit concentration, then - ? G ? Go RTln(Q) ? Go RTln(11 /
11) - ? G ? Go RTln(Q) ? Go RTln(1)
- or ? G ? Go RT x 0,
- or ? G ? Go,
- when all components are at 1
- .. a special case
- (when all components are at 1)
- 1 usually means 1 M
42
- So ? G and ? Go are quite different,
- and not to be confused with each other.
- ? Go allows us to compare all reactions under the
same standard reaction conditions that we all
agree to, independent of concentrations. - So it allows a comparison of the stabilities of
the bonds in the reactants vs. the products. - It is useful.
- AND,
- It is easily measured.
43
Because,
- at equilibrium, ? G ? Go RTln(Q) 0
- and at equilibrium Q Keq
- (a second special case).
- So at equilibrium, ? G ? Go RTln(Keq) 0
- And so ? Go - RTln(Keq)
- So just measure the Keq,
- Plug in R and T
- Get ?Go, the standard free energy change
44
E.g., lets say for the reaction A B C D,
Keq happens to be
- CeqDeq
- AeqBeq
- Then ? Go -RTlnKeq -2 x 300 x ln(2.5 x 10-3)
- -600
x -6 3600 - 3600 cal/mole (If we use R2 we are dealing with
calories) - Or 3.6 kcal/mole
- 3.6 kcal/mole ABSORBED (positive number)
- So energy is required for the reaction in the
left-to-right direction - And indeed, very little product accumulates at
equilibrium - (Keq 0.0025)
45
Note
- If ?Go 3.6 for the reaction A B lt --- gtC D
- Then ?Go -3.6 for the reaction C D lt--- gt A
B - (Reverse the reaction switch the sign)
- And
- For reactions of more than simple 1 to 1
stoichiometries - aA bB lt--gt cC dD,
- ?G ?Go RT ln CcDd
AaBb
46
Some exceptions to the 1M standard condition
Exception 1
- 1) Water 55 M (pure water) is considered unit
concentration instead of 1MThe concentration of
water rarely changes during the course of an
aqueous reaction, since water is at such a high
concentration. - So when calulating ?Go, instead of writing in
55 when water participates in a reaction (e.g.,
a hydrolysis) we write 1. - This is not cheating we are in charge of what is
a standard condition, and we all agree to this
55 M H20 is unit (1) concentration for the
purpose of defining ?Go.
47
Exception 2
- In the same way,
- Hydrogen ion concentration, H 10-7 M is
taken as unit concentration, by biochemists. - since pH7 is maintained in most parts of the cell
despite a reaction that may produce acid or base. - This definition of the standard free energy
change requires the designation ?Go - However, I will not bother.
- But it should be understood we are always talking
about ?Go in this course.
48
Summary
- ?G ?Go RTln(Q)
- This combination of one qualitative and one
quantitative (driving) term tell the direction of
a chemical reaction in any particular
circumstance - ?Go - RTln(Keq)
- The ?Go for any reaction is a constant that can
be looked up in a book.
49
- GOT THIS FAR
50
ATP, a small molecule in the cell that helps in
the transfer of energy from a place where it is
generated to a place where it is needed.
e
e
A-R
51
The hydrolysis of ATP
ADP
AMP
- ATP HOH ?? ADP Pi
- A adenine (2 rings with Ns)
- R ribose (5-carbon ring sugar)
The ?Go of this reaction is about -7
kcal/mole. Energy is released in this
reaction. This is an exergonic
reaction Strongly to the right, towards
hydrolysis, towards ADP
52
O O O
A-R-O-P-O-P-O-
-O-P-O-
O- O- O-
O O O
A-R-O-P-O-P-O-P-O- HOH
O- O- O-
ATP Adenosine triphosphate
ADP Adenosine diphosphate
Pi Inorganic phosphate
The ?Go of this reaction is about -7
kcal/mole. Energy is released in this
reaction. This is an exergonic
reaction Strongly to the right, towards
hydrolysis, towards ADP
53
High energy bonds
- ?Go of a least -7 kcal/mole is released upon
hydrolysis - Designated with a squiggle () often
- ATP A-P-PP
- Rationalized by the relief of electrical
repulsion upon hydrolysis
?Go -7 kcal/mole
54
- A-R-PPP
Not a high energy bond
ATP HOH
ATPase
? ADP Pi heat
Prob set 4
Keq 100,000
55
- The cell often uses the hydrolysis of ATP to
release energy. - The released energy is used to drive reactions
that require energy. - How does this work ??
56
E.g., A reaction that requires energy,an
endergonic reactionglucose Pi
glucose-6-phosphate
OP03--
Pi
Keq 2.5 x 10 -3
Glucose Pi --gt glucose-6-P H2O ? Go
3.6 kcal/mole.
ATP H2O --gt ADP Pi ? Go
-7 kcal/mole
Glucose Pi --gt G6P H2O ? Go
3.6 kcal/mole
ATP H2O Glucose Pi ? ADP Pi G6P H2O
Go -3.4 kcal/mole overall
Glucose ATP --gt G6P ADP
? Go -3.4 kcal/mole overall
net sum of the two considered
reactions
57
? Gos of multiple reactions are additive
- ATP H2O --gt ADP Pi ? Go
-7 kcal/mole - Glucose Pi --gt G6P H2O ? Go
3.6 kcal/mole - Glucose ATP --gt G6P ADP ? Go -3.4
kcal/mole overall net sum of the two
considered reactions - Enzymes needed ATPase? Glucose phosphorylase?
- No.
- Just get 7kcal/mole as heat.
- But
- Hexokinase
- Glucose AR-P-P-P ??glucose-6-P AR-P-P
- Glucose ATP ?? glucose-6-P04 ADP, ? Go
-3.4 kcal/mole - A coupled reaction. A new reaction, ATP not
simply hydrolyzed. - One of two ways the cell solves the problem
of getting a reaction to go in the desired
direction. The second later.
58
ATP
ATP
ATP
ATP
ATP
ATP
ATP
ATP
ATP
59
- So does this solve the direction problem? Only
for a second - Where does this ATP come from, if we are E. coli
growing in minimal medium - Glucose is the only carbon source.
- Need to make ATP from glucose, and this TAKES
energy. - But need only to regenerate ATP from ADP
Via GLYCOLYSIS, e.g.
Handout 7-1a
60
Regeneration of ATP from ADP
- Two solutions
- 1) Photosynthesis
- 2) Catabolism of organic comounds (e.g., glucose)
61
Glucose catabolism overview/preview
- 1- GLYCOLYSIS (6C ? 3C)
- 2- KREBS CYCLE (3C 1C, CO2 release)
- 3- ELECTRON TRANSPORT CHAIN (oxygen
uptake, water release) - Glycolysis, in detail, as
- Basic mechanism of energy metabolism(getting
energy by glucose breakdown.) - An example of a metabolic pathway.
62
Handout 7-2