PI Class Evolution - PowerPoint PPT Presentation
1 / 27
Title:
PI Class Evolution
Description:
HAART containing ritonavir-boosted protease inhibitor ... 4 boosted PIs as preferred PI therapy1: Atazanavir/r (IAS-USA only) Fosamprenavir/r ... – PowerPoint PPT presentation
Number of Views:52
Avg rating:3.0/5.0
Title: PI Class Evolution
1
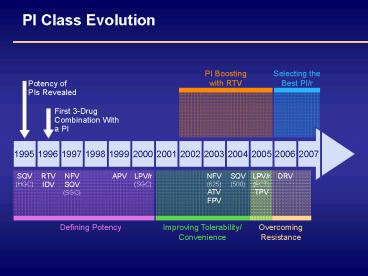
PI Class Evolution
Selecting theBest PI/r
PI Boostingwith RTV
Potency ofPIs Revealed
First 3-DrugCombination With a PI
1995
1997
1996
1999
1998
2001
2000
2003
2002
2005
2004
2006
2007
SQV(HGC)
RTVIDV
NFVSQV(SGC)
APV
LPV/r(SGC)
NFV(625)ATVFPV
LPV/r(FCT)TPV
SQV (500)
DRV
Improving Tolerability/Convenience
Defining Potency
OvercomingResistance
2
Contribution of the PI Class to ARV Therapy
- More PIs approved than any other class of ARV
- No other class has undergone a similar evolution
in reformulation to promote acceptability - Resistance is less common with the use of PI/r
- PIs are just as convenient, tolerable, and safe
as NNRTIs - New agents are effective against resistant virus
clinicaloptions.com/hiv
3
PI Studies Guiding Treatment Choices
GEMINI SQV/r vs LPV/r in Treatment-Naïve Patients
BASIC ATV/r vs SQV/r
SQV/r Potency of Boosted PIs Proven
2004
1998
2005
2007
KLEAN Kaletraor Lexiva WithRTV Combined With
Epivir And Abacavir In Naïve Patients
ALERT Once DailyfAPV/r vs ATV/r
4
Differentiating Boosted PIs in ARV-Naïve
Patients Comparative Study Results
- François Raffi
5
PI Selection for Treatment-Naïve Patients
- HAART containing ritonavir-boosted protease
inhibitor (PI/r) is recommended in first-line
treatment1,2 - European and international guidelines recommend
4 boosted PIs as preferred PI therapy1 - Atazanavir/r (IAS-USA only)
- Fosamprenavir/r
- Lopinavir/r
- Saquinavir/r
- Head-to-head comparative trials guide the
decision about PI selection - Three major attributes to consider
- Potency and durability
- Convenience
- Tolerability
1 IASUSA Guidelines, Hammer et al. JAMA
2006296827843. 2 The EACS Executive Committee
2005 http//www.eacs.ws/guide/m_guides.htm.
6
KLEAN Study Design
- N 882
- USA, Europe, Canada
- Duration 48 weeks
- Non inferiority design
- Inclusion criteria
- ARV-naïve
- HIV RNA 1,000 copies/mL
- Primary endpoint
- Proportion with VL lt400 copies/mL at week 48
- Proportion who permanently discontinued
randomized treatment due to adverse events
fAPV/r 700/100 mg bid ABC/3TC
11 randomization
LPV/r 400/100 mg bid ABC/3TC
Eron J et al. Lancet. 2006 368 476482.
7
KLEAN Virological Response
Patients with VL lt50 copies/mL at week 48
89
100
88
80
66
65
60
fAPV/r
LPV/r
40
20
n 434
n 444
n 328
n 341
0
ITT/e TLOVR
Observed
Safety data was comparable between the two
treatment arms
ITT/e All patients exposed to 1 dose of
randomized study medication. TLOVR Time to loss
of virological response.Eron J et al. Lancet.
2006368476482.
8
KLEAN Similar Lipid Increases with fAPV/r and
LPV/r
fAPV/r Baseline
LPV/r Baseline
Mean lipid values (mg/dL)
fAPV/r Week 48
LPV/r Week 48
250
200
150
100
50
0
HDL
LDL
Total Cholesterol
Triglycerides
11 of patients used lipid lowering therapy in
both arms.Eron J et al. Lancet. 2006368476482.
9
ALERT Study Design
- N 106
- Duration 48 weeks
- Open-label trial
- Inclusion criteria
- ARV-naïve
- HIV RNA 1,000 copies/mL
- Any CD4 count
- Primary endpoint
- VL lt50 copies/mL at week 48
- Secondary endpoints
- VL lt50 copies/mL at 24 weeks and lt400 copies/mL
at 24 and 48 weeks - Change from baseline in CD4 count
- Safety and resistance assessments
fAPV/r 1400/100 mg qd TDF/FTC
11 randomization
ATV/r 300/100 mg qd TDF/FTC
Smith K et al. 46th ICAAC 2006 Abstract H-1670a.
Investigational (qd dosage in US is fAPV/r
1400/200 mg)
10
ALERT Virological Response at 24 Weeks
Patients with VL lt50 copies/mL at week 24
100
83
79
75
50
25
n 53
n 53
0
fAPV/r
ATV/r
ITT, MD F analysis. Smith K et al. 46th ICAAC
2006, Abstract H-1670a.
11
ALERT Change in Lipids at 24 Weeks
fAPV/r Baseline
Median level (mg/dL)
fAPV/r Week 24
250
200
150
100
50
0
HDL
LDL
Total Cholesterol
Triglycerides
38
39
n
48
38
46
39
48
38
45
39
48
38
46
39
48
38
Smith K et al. 46th ICAAC 2006 Abstract H-1670a.
12
Gemini Study Design
- Prospective, phase IIIb, randomized,
multi-center, open-label, 2-arm study - N 337 (USA, Canada, Puerto Rico, France,
Thailand) - Duration 48 weeks
- Non-inferiority Design
- Inclusion criteria
- Treatment-naïve
- CD4 350 cells/mm3
- HIV RNA gt10,000 copies/mL
- Primary endpoint
- patients with HIV-1 RNA lt 50 copies/mL at week
48 - Planned interim analysis of all patients
completing 24 weeks - Week 48 data to be presented Q4 2007
SQV/r 1000/100 mg bid TDF/FTC
11 randomization
LPV/r 400/100 mg bid TDF/FTC
Raffi et al. Abstract WePeB027 Wednesday, 25 July
2007
Walmsley et al. Abstract TuPeB069. Tuesday, 24
July 2007
13
Gemini Baseline Characteristics
Raffi F et al. IAS 2007 Abstract WePeB027.
14
Gemini Time Course of HIV-1 RNA Suppression (ITT
Population)
SQV/r lt 50 copies/mL
LPV/r lt 50 copies/mL
Patients
100
69.9
80
69.0
60
40
20
0
Wk 8
Wk 16
Wk 4
Wk 2
Wk 12
Wk 20
Wk 24
SQV/r
n
166
166
166
166
166
166
166
LPV/r
n
171
171
171
171
171
171
171
Raffi F et al. IAS 2007 Abstract WePeB027.
15
Gemini Time Course of HIV-1 RNA Suppression (ITT
Population)
SQV/r lt 400 copies/mL
LPV/r lt 400 copies/mL
SQV/r lt 50 copies/mL
LPV/r lt 50 copies/mL
Patients
100
81.3
81.3
69.9
80
69.0
60
40
20
0
Wk 8
Wk 16
Wk 4
Wk 2
Wk 12
Wk 20
Wk 24
SQV/r
n
166
166
166
166
166
166
166
LPV/r
n
171
171
171
171
171
171
171
Raffi F et al. IAS 2007 Abstract WePeB027.
16
Gemini Median Change from Baseline in HIV-1
Viral Load (ITT Population)
HIV-1 RNA viral load(log10 copies/mL)
0.0
-1.0
-2.0
-3.0
SQV/r -3.4
LPV/r -3.5
-4.0
Wk 8
Wk 16
Wk 4
Wk 0
Wk 12
Wk 20
Wk 24
Wk 2
SQV/r
145
158
150
143
142
144
n
155
166
LPV/r
155
161
151
150
144
142
n
155
170
Raffi F et al. IAS 2007 Abstract WePeB027.
17
Gemini Median Change from Baseline in CD4
Lymphocyte Count (ITT Population)
D CD4 count(cells/mm3)
LPV/r 134
SQV/r 127
SQV/r
136
147
137
133
132
137
n
144
159
LPV/r
144
146
140
135
134
133
n
141
162
Raffi F et al. IAS 2007 Abstract WePeB027.
18
Gemini Discontinuations by Week 24
Not study drugrelated (boating accident
sepsis). One death was likely study
drugrelated (hepatic failure). Raffi F et al.
IAS 2007 Abstract WePeB027.
19
Gemini Rates of Grade III-IV Adverse Events
Patients
35
SQV/r (n163)
29
28
30
25
20
15
12
8
7
10
6
6
4
5
0
Diarrhea
Nausea
Vomiting
Total patients with 1 AE
Individual AEs reported in gt3 of patients
Multiple occurrences of the same adverse event in
one individual counted only once.Raffi F et al.
IAS 2007 Abstract WePeB027.
20
Gemini Virological Failures
Adherence
LPV/r
SQV/r
Raffi F et al. IAS 2007 Abstract WePeB027.
2 consecutive viral loads above 400 copies/mL on
or after week 16.
21
Gemini Smaller Change in Triglycerides from
Baseline with SQV/r
SQV/r Baseline
LPV/r Baseline
Mean lipid values (mg/dL)
SQV/r Week 24
LPV/r Week 24
73
225
24
12
180
12
135
11
8
90
11
11
45
0
HDL
LDL
Total Cholesterol
Triglycerides
Slim J et al. 8th International Congress on Drug
Therapy in HIV Infection, 2006. Abstract PL2.5.
22
Gemini Fewer Patients Reach Intervention Levels
for Cholesterol and Triglycerides with SQV/r
SQV/r Baseline (n159)
LPV/r Baseline (n165)
SQV/r Week 24 (n133)
LPV/r Week 24 (n130)
Patients
40
31
30
26
23
20
18
16
14
10
10
9
10
2.4
1.9
1.5
0
LDL(100 mg/dL)
Fasting cholesterol grade 1(200 mg/dL 5.2
mmol/L)
Fasting triglycerides grade 2(400 mg/dL 4.5
mmol/L)
Slim J et al. 8th International Congress on Drug
Therapy in HIV Infection, 2006. Abstract PL2.5.
23
BASIC Boosted ATV or SQV-Induced Lipid Changes
- N 120
- Multinational, multicenter
- Duration 48 weeks
- Inclusion criteria
- Treatment-naïve with treatment indication
- Outcomes
- Effects on lipid profile
- Effects on body fat distribution by objective
measures
SQV/r 2000/100 mg qd TDF/FTC
11 randomization
ATV/r 300/100 mg qd TDF/FTC
Investigational SQV/r 2000/100 mg qd dosage. The
approved dosing regimen is SQV/r 1000/100 mg bid.
24
Chelsea Westminster Study Minimal Impact of
SQV/r and ATV/r on Glucose Utilization
Glucose Disposal Rate mg/min/kg
Hyperinsulinemic euglycemic clamp in HIV
patients starting HAART
50
45
SQV/r
40
ATV/r
35
30
Baseline
Week 4
Jackson A et al, BHIVA, 2007.
25
Attributes of the Ideal PI for Naïve Patients
ATV/r is approved for naïve patients in the US.
qd dosing as approved or used in
investigational studies.
26
Acknowledgements
- Jonathan B. Angel, MD
- Christian Aquilina, MD
- Jean-François Bergmann, MD, PhD
- Robert Bolan, MD
- Philip Brachman, MD
- U. Fritz Bredeek, MD, PhD
- Jason Brunetta, MD
- Robert Catalla, MD
- Catherine Creticos, MD
- Charles P. Craig, MD
- Yasmine Debab, MD
- Edwin Dejesus, MD
- Pierre Dellamonica, MD
- Serge Dufresne, MD
- Joseph Gathe, Jr, MD
- François Raffi, MD
- Isabelle Ravaux, MD
- Kiat Ruxrungtham, MD
- Dominique Salmon, MD, PhD
- Anne Simon, MD
- Jihad Slim, MD
- Fiona M. Smaill, MD
- Christian Trepo, MD, PhD
- Benoit Trottier, MD
- Sharon Walmsley, MSc, MD
- Douglas J. Ward, MD
- Yazdan Yazdanpanah, MD, MSc
- David Zucman, MD
- The Roche study management team.
- Gilead for provision of Truvada.
27
(No Transcript)