Proteins - PowerPoint PPT Presentation
1 / 25
Title:
Proteins
Description:
Are the most structurally sophisticated molecules known ... (if pH, salt [ ], temp, etc. are altered, protein may unravel and lose native conformation ... – PowerPoint PPT presentation
Number of Views:31
Avg rating:3.0/5.0
Title: Proteins
1
Proteins
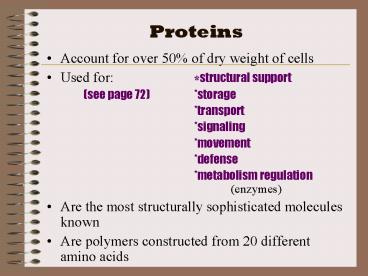
- Account for over 50 of dry weight of cells
- Used for structural support
- (see page 72) storage
- transport
- signaling
- movement
- defense
- metabolism regulation (enzymes)
- Are the most structurally sophisticated molecules
known - Are polymers constructed from 20 different amino
acids
2
Table 5.1 An Overview of Protein Functions
3
Hierarchy of structure
- Amino acids building blocks
- (are 20 different ones)
- Polypeptides polymers of amino acids
- Protein one or more polypeptides folded and
coiled into specific conformations.
4
General structure of amino acid
H
- All differ in the R-group
- The physical and chemical properties of the
R-group determine the characteristics of the
amino acid.
COOH
NH2
R
R
5
Nonpolar, Polar, and Electrically Charged
6
(No Transcript)
7
Amino Acids join
- Carboxyl group of one is adjacent to amino group
of another, dehydration synthesis occurs, forms a
covalent bond - PEPTIDE BOND
- When repeated over and over, get a polypeptide
- On one end is an N-terminus (amino end)
- On other is a C-terminus (carboxyl end)
8
Figure 5.16 Making a polypeptide chain
9
Proteins function depends on its conformation
- Functional proteins consist of one or more
polypeptides twisted, folded, and coiled into a
unique shape - Amino acid sequence determines shape
- 2 big categories 1. globular
- 2. Fibrous
- Function of a protein depends on its ability to
recognize and bind to some other molecule.
10
Lysozyme
11
Four levels of protein structure
- 1. Primary structure unique sequence of amino
acids (long chain) - 2. Secondary structure segments of polypeptide
chain that repeatedly coil or fold in patterns
that contribute to overall configuration - are the result of hydrogen bonds at
regular intervals along the polypeptide
backbone
12
Figure 5.18 The primary structure of a protein
13
Secondary structure Alpha helix and Beta
pleated sheet
- Helix delicate coil held together by H-bonding
between every fourth amino acid - Ex. Lysozyme, keratin
- Beta pleated sheet two or more regions of the
polypeptide chain lie parallel to one another.
H-bonds form here, and keep the structure
together. - Ex. Lysozyme, silk
14
Figure 5.20 The secondary structure of a protein
15
- 3. Tertiary structure superimposed on secondary
structure irregular contortions from
interactions between side chains - nonpolar side chains end up in clusters at the
core of a protein caused by the action of water
molecules which exclude nonpolar substances - (hydrophobic interaction)
- -van der Waals interactions, H-bonds, and ionic
bonds all add together to stabilize tertiary
structure - may also have disulfide bridges form (when amino
acids with 2 sulfhydryl groups are brought
together)
16
Figure 5.22 Examples of interactions
contributing to the tertiary structure of a
protein
17
- Quaternary Structure the overall protein
structure that results from the aggregation of
the polypeptide subunits - Ex. collagen structural
- hemoglobin globular
18
Figure 5.23 The quaternary structure of proteins
19
Figure 5.24 Review the four levels of protein
structure
20
What determines Protein configuration
- Polypeptide chain of given amino acid sequence
can spontaneously arrange into - 3-D shape
- Configuration also depends on physical and
chemical conditions of proteins environment - (if pH, salt , temp, etc. are altered,
protein may unravel and lose native conformation
- denaturation
21
Figure 5.25 Denaturation and renaturation of a
protein
22
Protein-Folding problem
- HOW proteins fold is not always clear may be
several intermediate states on the way to stable
conformation - Are a few ways to track, though
- chaperonins protein molecules that assist
the proper folding of other proteins. - Ex. From E. coli keeps polypeptide
segrated from problems in environment - computer simulations Blue Gene, a
supercomputer able to generate the 3-D
structure of any protein starting from its aa
sequence (medical uses)
23
Figure 5.26 A chaperonin in action
24
Chemical Tests for Protein ID
- For a liquid
- Biuret Reagent Test
- solutions containing a protein will turn
lavender/purple when combined with blue Biuret
Reagent - For a solid
- Xanthoproteic Test
- solids (that are light in color) will turn
yellow when exposed to nitric acid
25
Overconsumption of fat and protein