Clinical Trials Workflow - PowerPoint PPT Presentation
Clinical Trials Workflow
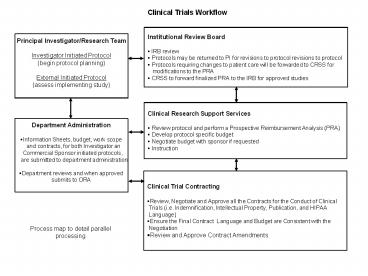
language for costs section of consent and contract negotiation for budgets ... and Budget are Consistent with. the Negotiation. Review and Approve Contract. Amendments ... – PowerPoint PPT presentation
Title: Clinical Trials Workflow
1
Clinical Trials Workflow
- Institutional Review Board
- IRB review
- Protocols may be returned to PI for revisions to
protocol revisions to protocol - Protocols requiring changes to patient care will
be forwarded to CRSS for - modifications to the PRA
- CRSS to forward finalized PRA to the IRB for
approved studies
Principal Investigator/Research
Team Investigator Initiated Protocol (begin
protocol planning) External Initiated
Protocol (assess implementing study)
- Clinical Research Support Services
- Review protocol and perform a Prospective
Reimbursement Analysis (PRA) - Develop protocol specific budget
- Negotiate budget with sponsor if requested
- Instruction
- Department Administration
- Information Sheets, budget, work scope
- and contracts, for both Investigator an
- Commercial Sponsor initiated protocols,
- are submitted to department administration
- Department reviews and when approved
- submits to ORA
- Clinical Trial Contracting
- Review, Negotiate and Approve all the Contracts
for the Conduct of Clinical - Trials (i.e. Indemnification, Intellectual
Property, Publication, and HIPAA - Language)
- Ensure the Final Contract Language and Budget
are Consistent with the - Negotiation
- Review and Approve Contract Amendments
Process map to detail parallel processing
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.