Enzyme Catalysis - PowerPoint PPT Presentation
1 / 22
Title:
Enzyme Catalysis
Description:
Enzymes have spectacular abilities to accelerate chemical reactions often by ... attacking group is at the other apex of the trigonal bipyramid (in-line attack) ... – PowerPoint PPT presentation
Number of Views:285
Avg rating:3.0/5.0
Title: Enzyme Catalysis
1
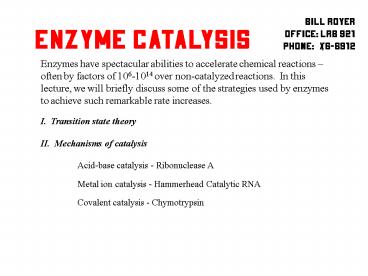
Bill Royer Office LRB 921 Phone x6-6912
Enzyme Catalysis
Enzymes have spectacular abilities to accelerate
chemical reactions often by factors of 106-1014
over non-catalyzed reactions. In this lecture,
we will briefly discuss some of the strategies
used by enzymes to achieve such remarkable rate
increases.
I. Transition state theory II. Mechanisms of
catalysis Acid-base catalysis - Ribonuclease
A Metal ion catalysis - Hammerhead Catalytic
RNA Covalent catalysis - Chymotrypsin
2
I. Transition state theory Consider the
reaction A B P Q where A B react
through transition state, X, to form products P
Q. K is the equilibrium constant between A
B and X and k' is the rate constant for
conversion of X to P Q.
The transition state, X, is metastable. (Unlike
a reaction intermediate, the transition state has
only a transient existence, like a pebble
balanced on a pin. By definition, a transition
state cannot be isolated.) The transition state
can be thought of as sharing some features of the
reactants and some features of the products.
That is, some bonds in the substrate are on their
way to being broken and some bonds in the product
are partially formed.
3
The transition state, X, is in rapid equilibrium
with reactants with equilibrium constant K.
DG, the activation energy, is the difference in
Gibbs free energy between the transition state,
X, and the reactants. Since K is an
equilibrium constant, the now familiar equation
applies
where T is the absolute temperature in degrees
Kelvin (C 273) and R is the gas constant
(1.98 cal / mol / degree). In other words, the
frequency with which reactants achieve the
transition state is inversely proportional to the
activation energy barrier between the two.
The observed rate of the reaction, kobs, will be
a function of the concentration of the reactants,
the rate of conversion of X to P Q, k', and
will decrease exponentially with an increase in
DG.
4
Thus, the smaller the difference in free energy
of the reactants and the transition state, the
faster the reaction proceeds. Enzymatic rate
accelerations are achieved by lowering the
activation barrier between reactants and the
transition state, thereby increasing the fraction
of reactants able to achieve the transition
state. Enzymes reduce the activation barrier by
destabilizing the ground state of enzyme-bound
substrates and products, by stabilizing the
transition state, and/or by introducing a new
reaction pathway with a different transition
state that has a lower free energy.
If a catalyst lowers the activation barrier by
DDG, the rate of the reaction is enhanced by the
factor e DDG/RT. Consequently, a ten-fold rate
enhancement requires that DDG 1.36 kcal/mole,
less than the energy of a single hydrogen bond.
(DDG RTln10 1.98 x 10-3 kcal/molK x
298Kln(10) 1.36 kcal/mol)
5
Imaginary enzyme ("stickase") designed to
catalyze "cleavage" (breaking) of a metal stick
(Nelson Cox, Lehninger Principles of
Biochemistry, 3rd ed., 2000)
6
For a reaction that involves several steps, each
step will have a corresponding transition state.
7
II. Mechanisms of catalysis A. Acid-base
catalysis Specific acid or base catalysis -
Reaction rate is directly proportional to H or
OH-. Example Alkaline hydrolysis of RNA
General acid or base catalysis - Reaction rate
is proportional to Bronsted acid or Bronsted
base Bronsted acid - species that can donate
protons Bronsted base - species that can
combine with a proton
8
Amino acids side chains with pKa's in the neutral
pH range can function as Bronsted acids/bases
9
Biologically important nucleophilic groups
Nucleophilic form
-
Hydroxyl group
H
-
H
Sulfhydryl group
R-NH3
R-NH2
H
Amino group
R
H
Imidazole group
N
HN
Adapted from Voet Voet, Biochemistry
10
Ribonuclease A An example of concerted acid-base
catalysis - reaction subject to both general acid
and general base catalysis
RNase A (124 residues, mw 13.7 kd) is a digestive
enzyme secreted by the pancreas that catalyzes
hydrolysis of phosphodiester backbone of RNA. In
first step of the reaction, cleavage of the bond
between phosphorous and the 5' oxygen generates
one 2',3'-cyclic phosphate terminus and one
5'-OH. In the second step, water reacts with the
cyclic phosphate to yield a 3' phosphate. The
2',3' cyclic phosphate can be isolated because it
forms more rapidly than it hydrolyzes.
11
First Step 23 cyclic nucleotide produced.
His 12 is general base, His 119 is general acid
12
Proposed mechanism of RNase A catalysis. The
unionized form of His 12 accepts a proton from
the 2' OH which enhances its nucleophilicity.
The protonated form of His 119 begins to donate
its proton to the 5' O, and the 2'O begins to
form a bond with P to form a pentacoordinate
transition state. The negative charge that
develops is stabilized electrostatically by the
nearby positively charged side chain of
lysine 41. The bond between P and the 5'-O
breaks when the proton from histidine 119 is
completely transferred. At the same time, a bond
between P and the 2'-O becomes fully formed,
producing the 2',3'-cyclic intermediate.
Hydrolysis of the cyclic intermediate is a
reversal of the first stage with H2O replacing
the 5'-O component that was removed. Histidine 12
is now the proton donor and histidine 119 is the
proton acceptor.
Geometry of the pentacovalent transition state.
The central phosphorus atom is transiently bonded
to 5 oxygen atoms. Three oxygens are coplanar
with the phosphorus. The oxygen atoms of the
leaving group is at one apex, and the oxygen atom
of the attacking group is at the other apex of
the trigonal bipyramid (in-line attack).
13
Evidence for RNase A mechanism pH dependence of
Vmax/KM for RNase A catalyzed hydrolysis of
cytidine-2',3'-cyclic phosphate. Bell shaped
curve suggests a catalytic role for functional
groups with pK's of 5.4 and 6.4, consistent with
histidines.
Crystal structure of RNase A complex with
cytidine 2'3'-cyclic phosphate intermediate.
Shows histidines and lysine appropriately
positioned in the active site. Note hydrogen
bonding interactions between cytosine and
threonine 45 that confer substrate specificity.
Chemical modification. Iodoacetate alkylates
histidine 119 or histidine 12 but not both in the
same molecule. Alkylation of either histidine
eliminates catalysis. Complex formation with
substrate or competitive inhibitors protects
histidines from modification.
14
B. Metal ion catalysis 1. Water ionization. A
metal ion's charge makes its bound water
molecules more acidic than free H2O and therefore
a source of OH- ions even below neutral pH (Metal
ions have been called "Super acids").
2. Charge shielding - metal ions can have charge
gt 1. 3. Oxidation-Reduction
15
- C. Covalent catalysis - Transient formation of a
catalyst-substrate covalent bond - Provides an alternative reaction pathway, with
two lower energy transition states - 1. A nucleophile (electron-rich group with a
strong tendency to donate electrons to an
electron-deficient nucleus) on the enzyme
displaces a leaving group on the substrate,
forming a covalent bond. - 2. The enzyme substrate bond decomposes to form
product and free enzyme. - Covalent catalyst must be a good nucleophile and
a good leaving group - highly mobile electrons
(imidazole of His, thiol of Cys, carboxyl of Asp,
hydroxyl of Ser). - Chymotrypsin, 25 kd serine protease, catalyzes
hydrolysis of proteins in the small intestine.
Chymotrypsin catalyzes hydrolysis of esters as
well as peptide bonds which has been useful for
analysis of the catalytic mechanism, although not
physiologically relevant.
16
o
Formation of the acyl-enzyme intermediate occurs
during the initial rapid phase and slower
hydrolysis (deacylation) of the acyl-enzyme
intermediate occurs during the second, slower
phase.
17
First stage in peptide bond hydrolysis
acylation. Hydrolysis of the peptide bond starts
with an attack by the oxygen atom of the Ser195
hydroxyl group on the carbonyl carbon atom of the
susceptible bond. The carbon-oxygen bond of this
carbonyl group becomes a single bond, and the
oxygen atom acquires a net negative charge. The
four atoms now bonded to the carbonyl carbon are
arranged as a tetrahedron. Transfer of a proton
from Ser195 to His57 is facilitated by Asp102
which (i) precisely orients the imidazole ring of
His57 and (ii) partly neutralizes the positive
charge that develops on His57 during the
transition state. The proton held by the
protonated form of His57 is then donated to the
nitrogen atom of the peptide bond that is
cleaved. At this stage, the amine component is
hydrogen bonded to His57, and the acid component
of the substrate is esterified to Ser195. The
amine component diffuses away.
18
Second stage in peptide hydrolysis deacylation.
The acyl-enzyme intermediate is hydrolyzed by
water. Deacylation is essentially the reverse of
acylation with water playing the role as the
attacking nucleophile, similar to Ser195 in the
first step. First, a proton is drawn away from
water. The resulting OH- attacks the carbonyl
carbon of the acyl group that is attached to
Ser195. As in acylation, a transient tetrahedral
intermediate is formed. His57 then donates a
proton to the oxygen atom of Ser195, which then
releases the acid component of the substrate,
completing the reaction.
19
Chymotrypsin catalytic triad Ser195/His57/Asp102
located at the active site by x-ray
crystallography. An important stabilizing feature
of the interaction between enzymes and their
substrates, is transition state binding. In
fact, most enzyme active sites are organized such
that binding to the transition state is preferred
over binding to either substrates or products.
The active site of chymotrypsin is arranged to
stably interact with the negatively charged
carbonyl oxygen of the tetrahedral intermediate
this part of the active site is referred to as
the oxyanion hole.
20
(No Transcript)
21
(No Transcript)
22
Mechanism of Protein Splicing The protein
splicing pathway consists of four nucleophilic
displacements. X represents the S or O atom of
the Cys/Ser/Thr sidechains. From Perler, FB
(1998) Cell 92, 1-4