Chapter 22. alpha Substitution of - PowerPoint PPT Presentation
1 / 53
Title:
Chapter 22. alpha Substitution of
Description:
B. Keto-enol tautomerism. 1. base catalyzed. keto. Chapter 22. 5. 2. acid ... 3. racemization: keto-enol tautomerism in action. Chapter 22. 7. Chapter 22. 8 ... – PowerPoint PPT presentation
Number of Views:418
Avg rating:3.0/5.0
Title: Chapter 22. alpha Substitution of
1
Chapter 22. alpha Substitution of Carbonyl
Compounds
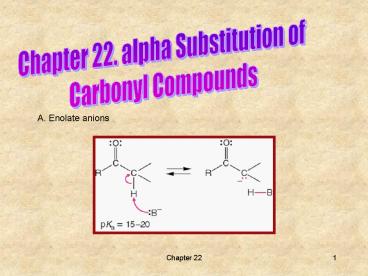
A. Enolate anions
2
resonance effect
inductive effect
3
(No Transcript)
4
B. Keto-enol tautomerism
1. base catalyzed
keto
5
2. acid catalyzed
6
3. racemization keto-enol tautomerism in action
7
(No Transcript)
8
4. keto-enol equilibrium constants
9
(No Transcript)
10
C. alpha-halogenation 1. base catalyzed a.
overall reaction
11
b. mechanism
etc
12
2. acid catalyzed a. general reaction
b. mechanism
13
The reaction stops after first halogenation
step.
c. examples
14
3. Hell-Volhardt-Zelinsky Reaction
(HVZ)
a. general reaction
b. partial mechanism
P Br2 ? PBr3
15
c. examples
NOTE
16
D. Alkylation of enolates 1. general reaction
LDA - our favorite choice for a base
17
2. orientation
18
BUT!!!!
So - use mild conditions ketones where only
one enolate can form
19
3. use of enamines a. formation
b. reactions
20
(No Transcript)
21
4. alkylation of esters
22
E. Aldol condensation 1. overall reaction
2. mechanism - base catalyzed
23
3. mechanism - acid catalyzed
24
4. getting it to work - a soxhlet extractor
?
?
25
(No Transcript)
26
5. retrosynthetic analysis
27
O
H2
O
H2
H2
O
28
6. intramolecular aldol reactions
Normally just five or six-membered rings can
be formed.
29
7. crossed Aldol reactions a. problem - can we
do this???
NO!! Four products are going to be formed.
30
b. no alpha hydrogens on one component
31
c. reactive aldehyde with no alpha hydrogen
d. use LDA to create enolate, then add reactive
carbonyl
32
F. Claisen condensation 1. general reaction
NOTE the base used is RO- - the alkoxy part of
the ester
2. mechanism
33
(No Transcript)
34
3. Dieckmann condensation - an intramolecular
variant of the Claisen
35
4. the crossed Claisen condensation and other
variants
36
Why this enolate?
37
(No Transcript)
38
G. Acetoacetic ester synthesis 1. general
reaction - alkylation step
2. general reaction - decarboxylation step
39
3. mechanism of decarboxylation step
40
4. examples
??
??
41
H. Malonic ester synthesis
42
I. Knoevenagel condensation
43
Retrosynthetic analysis
44
J. Conjugate addition - the Michael reaction 1.
general features 1,2 versus 1,4 addition
Which mode of reaction is going to be
observed??? NORMALLY 1,4 addition is
thermodynamically favored 1,2 addition is
kinetically favored
45
Remember from Chapter 15
Thermodynamic control
Kinetic control
The most stable product (C2) has the higher
activation barrier. So what happens now????
46
NORMALLY 1,4 addition is thermodynamically
favored 1,2 addition is kinetically
favored SO Weak or moderate nucleophiles prefer
1,4 - Michael addition Strong nucleophiles
(R-Li, H-, etc.) prefer 1,2 addition
NOTE
BUT
47
2. acid catalyzed mechanism for Michael addition
48
3. examples
nucleophiles that add 1,4
Michael electrophiles
49
Antitumor agent calicheamicin
Forces two alkynes groups close together
Abstracts H atoms from DNA
50
Note
51
4. Robinson annulation - a special class of
Michael additions which lead to one
six-membered ring fused to another
52
K. Summary 1.formation of enolate anions 2.
keto-enol tatomerism a. acid cat.
mechanism b. base cat. mechanism c.
structural effects on equilibrium 3.
alpha-halogenation a. acid cat. mechanism b.
base cat. Mechanism c. HVZ reaction -
mechanism 4. alkylation of enolates a.
orientation b. enamines c. alkylation of
esters 5. Aldol condensation a. acid base
cat. mechanism
53
b. retrosynthetic analysis c. intramolecular
reactions d. viable crossed Aldol reactions 6.
Claisen condensation a. mechamism b.
Dieckmann condensation c. viable crossed
Claisen reactions 7. Acetoacetic ester
synthesis a. mechanism b. decarboxylation 8.
Malonic ester synthesis - mech. 9. Knoevenagel
condensation - mech. 10. Michael addition a.
mechanism and acid cat. Variant b. reagents
favoring 1,4 and 1,2 additions c. Robinson
annulation - mech.