Phase behavior of two propoxylated surfactants
1 / 2
Title: Phase behavior of two propoxylated surfactants
1
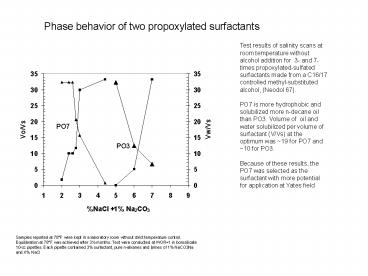
Phase behavior of two propoxylated surfactants
Test results of salinity scans at room
temperature without alcohol addition for 3- and
7-times propoxylated-sulfated surfactants made
from a C16/17 controlled methyl-substituted
alcohol, (Neodol 67). PO7 is more hydrophobic
and solubilized more n-decane oil than PO3.
Volume of oil and water solubilized per volume
of surfactant (V/Vs) at the optimum was 19 for
PO7 and 10 for PO3. Because of these results,
the PO7 was selected as the surfactant with more
potential for application at Yates field
Samples reported at 78F were kept in a
laboratory room without strict temperature
control. Equilibration at 78F was achieved
after 3½ months. Test were conducted at WOR1 in
borosilicate 10-cc pipettes. Each pipette
contained 3 surfactant, pure n-alkanes and
brines of 1 NaCO3Na and X NaCl
2
Phase behavior PO3 and PO7 surfactants vs n-
Alkanes
Optimal salinity vs. Alkane Carbon Number (ACN).
The number in parenthesis indicates
solubilization parameter at optimal
conditions. It was observed that PO3 produced a
bi-refringent middle phase against n-decane while
some bi-refringence was present in PO7 against
n-dodecane (V/Vs 10). PO7 samples at higher
temperatures up to 140F failed to produce any
bi-refringence. When increasing temperature, we
were able to clearly determine, from
well-equilibrated samples, that the optimal
salinity decreased, or lipophilicity increased.
For example, an under-optimum microemulsion at
3.7 NaCl _at_ 78F was an over-optimum
microemulsion at 140F. Because of lack of
equilibration for 2 month of monitoring samples,
we were unable to precisely determine optimal
conditions at 140F. We hypothesize that the
appearance of bi-refringence, as the oil
molecular was increased, is a micelles
manifestation of the surfactant lipophile not
being quite long-enough to solubilize more oil
with higher molecular weight. For PO-3
birefringence started at n-Decane where as for
PO-7 started at nDodecane. Because the
propoxylated surfactants become more lipophilic
when raising test temperature, the absence of
birefringence could help to validate this
hypothesis. This matter needs further
investigation.
Samples reported at 78F were kept in a
laboratory room without strict temperature
control. Equilibration at 78F was achieved
after 3½ months. Test were conducted at WOR1 in
borosilicate 10-cc pipettes. Each pipette
contained 3 surfactant, pure n-alkanes and
brines of 1 NaCO3Na and X NaCl