Alkynes: Be sure to know these - PowerPoint PPT Presentation
1 / 15
Title:
Alkynes: Be sure to know these
Description:
Alkynes: Be sure to know these & their mechanisms. Hydrohalogenation ... Steric hindrance around the leaving group causes 2 and 3 alkyl halides to ... – PowerPoint PPT presentation
Number of Views:56
Avg rating:3.0/5.0
Title: Alkynes: Be sure to know these
1
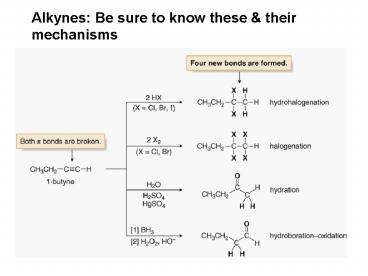
Alkynes Be sure to know these their mechanisms
2
HydrohalogenationElectrophilic Addition of HX
3
HalogenationAddition of Halogen
4
HydrohalogenationElectrophilic Addition of HX
5
HydrohalogenationElectrophilic Addition of HX
6
HalogenationAddition of Halogen
7
HydrationElectrophilic Addition of Water
- Internal alkynes undergo hydration with
concentrated acid, whereas terminal alkynes
require the presence of an additional Hg2
catalystusually HgSO4to yield methyl ketones by
Markovnikov addition of water.
8
HydrationElectrophilic Addition of Water
9
HydrationElectrophilic Addition of Water
10
HydroborationOxidation
Hydroborationoxidation is a two step reaction
sequence that converts an alkyne to a carbonyl
compound.
11
HydroborationOxidation
12
Introduction to Alkyne ReactionsAcetylide anions
13
Reactions of Acetylide Anions
14
Reactions of Acetylide Anions
- Steric hindrance around the leaving group causes
2 and 3 alkyl halides to undergo elimination
by an E2 mechanism, as shown with
2-bromo-2-methylpropane. - Nucleophilic substitution with acetylide anions
forms new carbon-carbon bonds in high yield only
with unhindered CH3X and 1 alkyl halides.
15
Reactions of Acetylide Anions
- Acetylide anions are strong nucleophiles that
open epoxide rings by an SN2 mechanism. - Backside attack occurs at the less substituted
end of the epoxide.