Solids - PowerPoint PPT Presentation
1 / 82
Title:
Solids
Description:
Instantaneous weak forces that hold one molecule to another or to another ... Smectic. Liquid crystal that have a well defined crystal structure in 2 dimensions ... – PowerPoint PPT presentation
Number of Views:48
Avg rating:3.0/5.0
Title: Solids
1
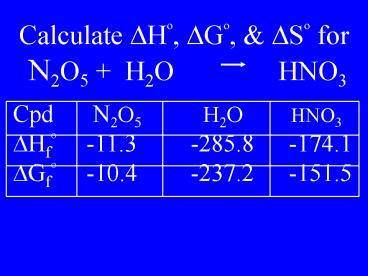
Calculate DHo, DGo, DSo for N2O5 H2O HNO3
Cpd N2O5 H2O HNO3 DHfo -11.3
-285.8 -174.1 DGfo -10.4 -237.2
-151.5
2
AP Chm HW
- Problems 7 9
- Page 268
3
Solids
4
Molecular Solids
- Covalently bound molecules held together by
intermolecular forces
5
Molecular Solids
- Non-conductors
- Insoluble in water mostly
- Low MP BP
- Held by intermolecular F
6
Intermolecular Forces
- Instantaneous weak forces that hold one molecule
to another or to another part of itself
7
(No Transcript)
8
Intermolecular Forces
- H-bond
- Dipole-dipole
- Dipole-induced dipole
- London dispersion
9
(No Transcript)
10
(No Transcript)
11
Network Molecular Solids
- Bound by a continuous network of covalent bonds
- High MP, insoluble, non-conductor
12
Ionic Solids
- Ions or ionic compounds held together by
electrostatic charge - Fattraction Kq1q2/d2
13
Ionic Solids
- Non-conductors as solids
- Conductors in solution
- Soluble in water mostly
- Very high MP BP
- Brittle
14
Metallic Solids
- Conductors, insoluble in water mostly, high MP
BP, held by gravitational type force - Fattraction Gm1m2/d2
15
Crystal
- Solid
- Any substance that has a well defined crystal
structure
16
Crystal Lattice
- The three dimensional arrangement of unit cells
in a crystal structure
17
Unit Cell
- The smallest repeating unit that a crystal
structure can be divided into
18
(No Transcript)
19
Drill
- Describe unit cells crystal lattice
20
Crystal Structures
- Cubic Monoclinic
- Tetragonal Triclinic
- Orthorhombic
- Hexagonal Rhombohedral
21
Cubic
- All angles 90o
- All sides are
- All faces are squares
22
Tetragonal
- All angles 90o
- 2 side sets are , third ?
- 1 set of opposing squares
- 2 sets opposing rectangles
23
Orthorhombic
- All angles 90o
- all 3 side sets are ?
- 3 unequal sets opposing rectangles
24
Hexagonal
- All angles 90o or 120o
- 1 set of opposing hexagons
- 3 sets opposing rectangles
25
Monoclinic
- 2 sets of angles 90o
- third set ?90o
- 1 set of opposing parallel
- 2 sets opposing rectangles
26
Triclinic
- No angles 90o
- 3 unequal sets of opposing parallelograms
27
Rhombohedral
- No angles 90o
- All sides
- 3 sets of opposing congruent rhombuses
28
Simple Cube
- Unit cell with one atom at each vertex
- 1 atom/cell
29
Body Centered Cube
- BCC
- Unit cell with one atom at each vertex one atom
in the center of the cube - 2 atoms/cell
30
(No Transcript)
31
Face Centered Cube
- FCC or CCP
- Unit cell with one atom at each vertex one atom
on each face of the cube - 4 atoms/cell
32
(No Transcript)
33
- The density of iron in its normal state of BCC is
7.86 g/mL. - Calculate its density in the FCC state
34
Drill
- List describe the 7 crystal structures
35
Hydrated Crystal
- A solid with water in the crystal
- CuSO45H2O
36
Anhydrous Solid
- A crystal without water
37
Hygroscopic
- Crystals that absorb moisture from the air
38
Deliquescent
- Crystals that absorb enough moisture from the air
to liquify
39
Efflorescent
- Crystals that give up water to the surroundings
40
Polymorphous
- When a single substance can have multiple crystal
structures
41
(No Transcript)
42
Isomorphous
- When different substances have the same crystal
structure
43
Amorphous Solid
- A solid w/o a well defined crystal structure
- Super-cooled liquid
44
Liquid Crystals
- Part solid part liquid
- Has a well defined crystal structure in 1 or 2
but not all 3 dimensions
45
Smectic
- Liquid crystal that have a well defined crystal
structure in 2 dimensions
46
Nematic
- Liquid crystals that have a well defined crystal
structure in only 1 dimension
47
(No Transcript)
48
Liquid
- A substance that holds together loosely, but has
no structure in any dimension
49
Solid
- Definite size shape
- Particles vibrate about fixed points
50
Liquid
- Definite size but no shape
- Particles vibrate about moving points
51
Gas
- No definite size or shape
- Particles move at random
52
(No Transcript)
53
Drill
- Name describe each of the 7 crystal structures
54
Melting Point
- Temperature at which the solid phase liquid
phase are at equilibrium - MP FP are equal
55
Melting Point
- Temperature at which the vapor pressure of a
solid the vapor pressure of its liquid phase
56
Boiling Point
- Temperature at which the liquid phase gaseous
phase are at equilibrium
57
Boiling Point
- Temperature at which the vapor pressure of a
liquid the vapor pressure of its gaseous phase
or atmospheric P
58
Adhesion
- The attraction of particles from different
substances to each other
59
Cohesion
- The attraction of particles of the same substance
towards each other
60
Capillarity
- The movement of a liquid up a thin tube due to
adhesion cohesion
61
Surface Tension
- Pressure on the surface of a liquid caused by the
uneven forces acting on the surface molecules
62
(No Transcript)
63
Vapor Pressure
- The pressure caused by the evaporated particles
in the vapor above a liquid
64
Intermolecular Forces
- Weak temporary attractions between atoms from one
molecule to another or another part of a larger
molecule
65
(No Transcript)
66
Intermolecular Forces
- Hydrogen-bond
- Dipole-dipole
- Dipole-induced dipole
- London dispersion forces
67
Hydrogen Bond
- Strongest of the intermolecular forces
- Occurs when H is bound to one highly EN element
connects to another
68
Dipole-Dipole
- When two polar molecules connect
69
Dipole-Induced Dipole
- When a polar molecule gets near a non-polar one,
it induces the non-polar one to become polar
thus, they connect
70
London Dispersion
- Instantaneous attraction for fractions of seconds
in which non-polar molecules connect - Very weak force
71
(No Transcript)
72
Predict explain the MP trends of1) Li,
Na, K, Rb2) F2, Cl2, Br2, I2 3) LiF, NaCl,
KBr, RbI
73
Phase Diagram
- Graphic representation of all the phases of a
substance with respect to temperature pressure
74
(No Transcript)
75
Approximate MP BP
1 atm
100 K
400 K
76
Phase Diagrams
77
(No Transcript)
78
Describe conditions at each number
79
AP Chm HW
- Problems
- 27, 51, 53
- Pages 269 270
80
Define solids, liquids, gases, melting Boiling
points
81
Determine the phase changes for the 3 arrows
82
(No Transcript)