Water Treatment - PowerPoint PPT Presentation
1 / 77
Title:
Water Treatment
Description:
... plants use quicklime (CaO) instead of hydrated lime (Ca(OH) ... Quicklime can easily be converted to hydrated lime by adding it to water in forming a slurry. ... – PowerPoint PPT presentation
Number of Views:2435
Avg rating:3.0/5.0
Title: Water Treatment
1
Water Treatment
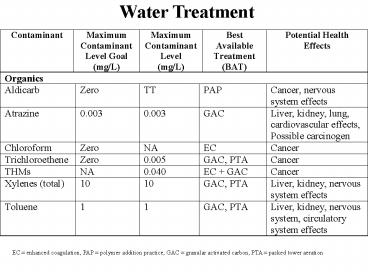
EC enhanced coagulation, PAP polymer addition
practice, GAC granular activated carbon, PTA
packed tower aeration
2
Water Treatment
3
(No Transcript)
4
Definitions
- Coagulation - The driving together of small or
colloidal particles by chemical forces into
larger ones. Rapid mixing is used and reaction
occurs very fast. - Flocculation - The assembling of coagulated
particles. Flocculation basins are used to
provide slow mixing.
5
NATURE OF COLLOIDAL PARTICLES IN WATER
- 1) Size - Very Small 1 - 1000 nm (sometimes to
10,000 nm) - 10 - 10000 Angstroms
- 10-4 - 10-7 cm
- 2) Surface Area - Very Large
- Diameter of Spheres Surface Area
- 1 cm 0.487 in2
- 10-4 cm 33.8 ft2
- 10-5 cm 3.8 yd2
- 10-6 cm 0.7 acres
- 10-7 cm 7 acres
- 3) Charge - usually negative
- - Repulsion of similar charges
- - Cause of colloidal stability
6
Size spectrum of waterborne particles
7
Purpose of Coagulation and Flocculation
- 1) Removal of colloidal organic and inorganic
particles that produce color, and retain
bacterial and viral organisms. - 2) Removal of metal ions that may cause hardness,
scaling, toxicity, taste and odor, etc - 3) Increase removal of suspended particulate
matter and BOD in primary settling basins. - 4) Improve performance of secondary settling
tanks following biological treatment processes. - 5) Can be used in the pretreatment of
contaminated groundwater prior to organic matter
removal.
8
Chemistry of Coagulation
- Primary Functions of Coagulant Chemicals
- 1 ) particle destabilization
- 2 ) strengthening of flocs to reduce floc breakup
- Selection of the Type of Coagulant Depends on
- 1) characteristics of the coagulant
- 2) particles
- 3) water quality
- 4) cost
- 5) dewatering characteristics of the solids
produced
9
Chemistry of Coagulation
- Types of Coagulants
- 1) Aluminum Sulfate (Al2(SO4)3 ? 14.3 H2O)
- 2) Ferric Sulfate (Fe2(SO4)3) or (FeCl3)
- 3) Lime (CaO)
- 4) Cationic Polymer
- 5) Anionic Polymer
- 6) Nonionic Polymer
10
Coagulant Chemicals
- Alum (aluminum Sulfate), Al2(SO4)314.3H2O is the
most common coagulant in the United States and is
often used in conjunction with cationic polymers. - Polyaluminum chloride, Al(OH)x(Cl)x, is efficient
in some waters requiring less pH adjustment and
producing less sludge. - Ferric chloride, FeCl3, may be more effective
than alum in some applications. - Ferric Sulfate, Fe2(SO4)2, is effective in some
waters and more economical in some locations. - Cationic polymers can be used alone as the
primary coagulant or in conjunction with aluminum
or iron coagulants.
11
Application of Fe (III) and Al(III) Salts for
Effective Coagulation
- The aquometal ions of aluminum and iron are
acidic in nature
If these aquometal species are acidic in water,
what happens to the alkalinity of the water?
12
Application of Fe (III) and Al(III) Salts for
Effective Coagulation
- Hydrogen ions are liberated by the addition of
alum and will react with the waters natural
alkalinity.
Theoretically, each mg/L of alum will consume
approximately 0.50 mg/L (as CaCO3) of alkalinity
and produce 0.44 mg/L of carbon dioxide. If the
natural alkalinity is not sufficient to react
with the alum and buffer the pH, it may be
necessary to add alkalinity to the water in the
form of lime or soda ash.
13
Application of Fe (III) and Al(III) Salts for
Effective Coagulation
- Stochiometric Equations for lime and soda ash
addition
Lime
Soda Ash
14
Water Treatment - Jar Testing Procedure
- Example - Jar tests can be used to evaluate the
coagulation efficiency of a coagulant. The
Phipps-Bird jar testing apparatus is recommended
for use. It consists of six 1-Liter beakers or
2-Liter square jars and a gang mixer. A jar test
is performed by first adding the same alum dose
and varying the pH in each jar. The test can be
repeated by holding the pH and varying the
coagulant dose.
15
Water Treatment - Jar Testing Procedure
16
Water Treatment - Jar Testing Procedure
17
Water Treatment - Jar Testing Procedure
- Example - In this example, two sets of jar tests
were conducted on a raw water containing 15 NTU
and an HCO3- alkalinity of 50 mg/L expressed as
CaCO3. Given the data below, find the optimal pH,
coagulant dose, and the theoretical amount of
alkalinity that would be consumed at the optimal
dose.
18
Water Treatment - Jar Testing Procedure
- Solution The above results are plotted in the
following figures.
19
Water Treatment - Jar Testing Procedure
- pH
20
Water Treatment - Jar Testing Procedure
- Alum Dose, mg/L
21
Water Treatment - Jar Testing Procedure
- Solution Base on the plots of the data, the
optimal pH was chosen as 6.25 and the optimal
alum dosage was about 12.5 mg/L. The experiments
may be repeated using a pH of 6.25 and varying
the alum dose between 10 and 15 mg/L to pinpoint
the optimal conditions. - The amount of alkalinity that will be consumed is
found by using the stochiometric expressions
which shows that one mole of alum consumes six
moles of HCO3- alkalinity.
22
Water Treatment - Jar Testing Procedure
- Solution The quantity of alkalinity that will
be consumed is found by using the following
stochiometric expression.
Using the above equation, and a molecular weight
of 594 for alum, the amount of alkalinity
consumed is calculated as
23
Water Treatment - Jar Testing Procedure
- Solution
24
Water Treatment - Jar Testing Procedure
- Equivalent weight concept see Page 138 in
Textbook. - In an acid/base reaction, n is the number of
hydrogen ions that the molecule transfers. That
is, an acid gives up an EW of hydrogen ions, and
a base accepts an EW of hydrogen ions. - In a precipitation reaction, n is the valence of
the element in question. For compounds, n is
equal to the number of hydrogen ions the would be
required to replace the cation that is for
CaCO3, it would take two hydrogen ions to replace
the calcium, therefore, n 2. - In a oxidation/reduction reaction, n is equal to
the change in oxidation number that the compound
undergoes in the reaction.
25
Hardness in Water
- Hardness is a term used to characterize a water
that does not lather well, causes a scum in the
bath tub, leaves hard, white, crusty deposits
(scale) on coffee pots, tea kettles, and hot
water heaters, and deteriorates fabrics.
Soap will be consumed by the polyvalent cations
first and will not interact effectively with dirt
particles on clothing, and the calcium-soap
complex forms undesirable precipitates.
26
Hardness - Regional Variation
- In the U.S., there is significant regional
variation in hardness of both surface water and
groundwater
27
Hardness - Guidelines
- The maximum levels of hardness permitted in a
public water supply is 300 to 500 mg/L. - However, most people will object to having water
harder than about 120 mg/L. - Typical Considerations of Total Hardness
- Low 0-75 mg/L
- Moderate 75-150 mg/L
- Moderately Hard 150-200 mg/L
- Very Hard gt 200 mg/L
28
Hardness Formation in Water
29
Hardness - Classifications
- Hardness is defined as the sum of all polyvalent
cations. The common units of expression are in
mg/L as CaCO3 of meq/L. - The predominant polyvalent cations in drinking
water supplies are calcium (Ca2) and magnesium
(Mg2) ions. Consequently, the main focus for
most water softening applications is the removal
of calcium and magnesium ions.
30
Hardness - Classifications
- Total Hardness
- Sum of Magnesium and Calcium Ions
- Total Hardness (TH) Carbonate Hardness (CH)
Noncarbonate Hardness (NCH) - Carbonate Hardness
- Caused by cations from the dissolution of calcium
or magnesium carbonate and bicarbonate in the
water (chemically equivalent to alkalinity) - Noncarbonate Hardness
- Caused by cations from calcium and magnesium
compounds of sulfate, chloride, or silicate
dissolved in the water.
31
Hardness Bar Charts
- Bar charts are useful in understanding and
characterizing constituents in water. - A conventional bar chart is constructed with
cations in the upper bar and anions in the lower
bar. - In the upper bar, calcium is placed first
followed by magnesium. Other cations are placed
after in no particular order. - In the lower bar bicarbonate is placed first
followed by other anions without any specific
order.
32
Hardness Bar Charts
- Example 3-11. Given the following analysis of a
groundwater, construct a bar chart of the
constituents expressed as CaCO3.
33
Hardness Bar Charts
- Example 3-11 Continued
34
Hardness Bar Charts
- Example 3-11 Continued
- Note that there is a discrepancy between the
total cations and anions. The cations total is
316 (as CaCO3) as compared to anions total of 312
mg/L (as CaCO3). This is probably because the
there are other ions that were not analyzed. If
complete analysis were conducted, and no
analytical error occurred, the equivalents of
cations would exactly equal the equivalents of
anions. Typically, a complete analysis may vary
by about 5 because of analytical error.
35
Hardness Bar Charts
- Example 3-12.
- A water has an alkalinity of 200 mg/L as CaCO3.
The Ca2 concentration is 160 mg/L as the ion,
and the Mg2 concentration is 40 mg/L as the ion.
The pH is 8.1. Find the total, carbonate and
noncarbonate hardness.
36
Hardness Bar Charts
- Example 3-12. Solution.
- Molecular weights Ca2 40 Mg2 24
- Valence Ca2 2 Mg2 2
- Equivalent weights
- Ca2 40/2 20 mg/meq
- Mg2 24/2 12 mg/meq
37
Hardness Bar Charts
- Example 3-12. Solution.
- By definition, the carbonate hardness is the
lesser of the total hardness or the alkalinity.
In this case, the alkalinity is less than the
total hardness, and the carbonate hardness is
equal to the alkalinity CH 200 as CaCO3. The
noncarbonate hardness is equal to the difference
between the TH and CH as
38
Lime Softening - Process Chemistry
- In lime softening it is possible to calculate the
chemical doses necessary to remove hardness.
Hardness precipitation is based on the following
two solubility reactions
and
The objective is to precipitate calcium as CaCO3
and the magnesium as Mg(OH)2. Precipitating
calcium as CaCO3 requires the pH of the water to
be raised to about 10.3. Precipitating
magnesium requires the pH of the water to be
raised to about 11.
39
Lime Softening - Process Chemistry
- When there is not enough natural bicarbonate
alkalinity (HCO3-) present in the water for
CaCO3 (s) precipitate to form (noncarbonate
hardness), carbonate must be added to the water. - Magnesium is more expensive to remove than
calcium, so many times Mg2 is left in the water.
This is called selective calcium removal. - Noncarbonate hardness is more expensive to remove
than carbonate hardness because another chemical
needs to be added to provide carbonate.
40
Lime Softening - Process Chemistry
- The chemical process used to soften water are a
direct application of the law of mass action.
Increasing the concentration of carbonate and/or
hydroxide by the addition of chemicals will drive
the reactions shown in Eqs 3-58 and 3-59 to the
the right. - Typically the pH of natural water is around 6 -
8 and the alkalinity exists mostly as
bicarbonate. - The addition of hydroxide or carbonate ions
causes the carbonate buffer system to move to the
right as well as an increase in pH.
41
Bicarbonate and Carbonate Species as f (pH)
42
Lime Softening - Process Chemistry
- The most common source of hydroxide ions is
calcium hydroxide Ca(OH)2. Many water treatment
plants use quicklime (CaO) instead of hydrated
lime (Ca(OH)2 because of economics. - Quicklime can easily be converted to hydrated
lime by adding it to water in forming a slurry.
This is called slaking.
This reaction is highly exothermic and must be
controlled carefully (1 MJ per gram mole of
lime). When we speak of adding lime we mean
adding calcium hydroxide Ca(OH)2.
43
Hardness TreatmentLime - Soda Ash Softening
- Precipitation softening removes the calcium and
magnesium from the water using - Unslaked Lime (CaO) or Slaked Lime (Ca(OH)2)
- Soda Ash (Na2CO3)
- Added benefits of this treatment
- Bactericidal action - kills bacteria, viruses,
algae - Removal of iron, manganese, mercury, chromium
- Aid in turbidity and NOM (THMFP) removal
- Reduces pipe corrosion and prevents scale in
boilers
44
Hardness TreatmentLime - Soda Ash Softening
- Lime (CaO) is sold commercially in forms of
quicklime and hydrated lime. - Quicklime
- Granular in form
- Usually greater than 90 CaO with magnesium oxide
being the primary impurity - Usually crushed in a slaker and fed to slurry
containing about 5 calcium hydroxide - Hydrated Lime
- Powdered and contains about 70 percent CaO
- Prepared by fluidizing in a tank containing a
turbine mixer - Lime slurry is written as Ca(OH)2.
45
Hardness TreatmentLime - Soda Ash Softening
- Soda Ash
- Grayish-white powder
- Nearly 98 percent sodium carbonate
- Carbon Dioxide
- Clear, colorless gas
- Used for recarbonation, which lowers the pH and
stabilizes the lime-softened water - Recarbonation prevents deposition of hard
carbonate scale on filter sand and distribution
piping
46
Lime Softening Softening Reactions
- Softening reactions are regulated by controlling
the pH. First any free acids in the water are
neutralized. Then the pH is raised to
precipitate CaCO3 if necessary, the pH is raised
further to remove magnesium. Also if necessary,
carbonate is added to precipitate noncarbonate
hardness. The following six important softening
reactions are presented below
47
Lime Softening Softening Reactions
- Neutralization of carbonic acid (H2CO3).
- In order to raise the pH, all the free acids
present in the water must be neutralized. CO2
(or H2CO3) is the principal acid present in
natural waters. Note that no hardness is removed
in this reaction.
48
Lime Softening Softening Reactions
- Precipitation of carbonate hardness due to
calcium. - To precipitate calcium as calcium carbonate, the
pH must be raised to 10.3 Adding lime raises
the pH and converts all the bicarbonate
alkalinity to carbonate. The carbonate then
serves as the common ion for the precipitation
reaction.
49
Lime Softening Softening Reactions
3. Precipitation of carbonate hardness due to
magnesium. If carbonate hardness due to
magnesium is to be removed, enough lime must be
added to increase the pH to about 11. The
reaction for magnesium may occur in two stages.
The first stage occurs when all the bicarbonate
is converted to carbonate.
50
Lime Softening Softening Reactions
3. Precipitation of carbonate hardness due to
magnesium. With the addition of more lime the
hardness due to magnesium is removed.
51
Lime Softening Softening Reactions
- Removal of noncarbonate hardness due to calcium.
- If noncarbonate hardness is to be removed due to
calcium, no further increase in pH is required.
Instead additional carbonate (alkalinity) is
added in the form of soda ash.
52
Lime Softening Softening Reactions
5. Removal of noncarbonate hardness due to
magnesium. If noncarbonate hardness is to be
removed due to magnesium, both lime and soda ash
need to be added. The lime provides the
hydroxide ion for precipitation of the magnesium.
53
Lime Softening Softening Reactions
5. Removal of noncarbonate hardness due to
magnesium. In Eq. 3-66, hardness was not
removed as Ca2 replaced Mg2. To remove calcium
soda ash must be added.
54
Lime Softening Softening Reactions
55
Lime Softening Process Limitations
- Minimum calcium hardness that can be achieved is
30 mg/L as CaCO3. - Minimum magnesium hardness that can be achieved
is 10 mg/L as CaCO3. - Some municipalities prefer to soften the water to
about 75 mg/L as CaCO3 because a very soft water
results in a slimy feeling when using soap. - Magnesium in excess of about 40 mg/L as CaCO3
forms scales on heat exchange elements in hot
water heaters. Because of the expense in
removing magnesium, it is normally removed if it
in excess of 40 mg/L as CaCO3.
56
Lime Softening Process Limitations
- For magnesium removal an excess of lime equal to
20 to 40 mg/L as CaCO3 is added to provide an
increase in the pH and improve kinetics of the
reaction. About 40 mg/L as CaCO3 is typically
used.
57
Lime Softening Chemical Additions
- The chemical additions (as CaCO3) to soften water
may be summarized as follows
aThe terms lime and Soda refer to mg/L
of Ca(OH)2 and Na2CO3 as CaCO3 equal to mg/L of
ion as CaCO3
58
Lime Softening Softening Reactions
59
Depending upon the softening process utilized,
the treated water will usually have a pH of 10 or
greater. Consequently, it is necessary to lower
the pH and stabilize the water to prevent the
deposition of hard carbonate scale on filter sand
and distribution piping. In this process carbon
dioxide is added to water in sufficient quantity
to lower the pH to within the range of 8.4 to
8.6. When magnesium is removed from the water,
excess lime is added to raise the pH above 11 to
precipitate magnesium hydroxide. In this case
enough CO2 must be added to to neutralize the
excess hydroxide ions as well as to convert the
carbonate ions to bicarbonate ions. These
calculations are discussed in CE4508.
Lime Softening Recarbonation
60
Example 3-13 From the water analysis presented
below, determine the amount of lime and soda (in
mg/L as CaCO3) necessary to soften the water to
80.00 mg/L hardness as CaCO3. Calculate the
quantity of CaCO3 solids produced if the design
flow is 100 ML/d.
Water Composition (mg/L) Ca2 95.20 Mg2
13.44 Na 25.76 CO2 19.36 HCO3-
241.46 SO42- Cl- 67.81
61
Example 3-13 SolutionSet up table and convert
the ion concentrations to CaCO3 equivalents.
62
Example 3-13 bar chart
63
Example 3-13 Solution
- Based on the bar chart
- CO2 does not contribute to the hardness
- TH 293.37 mg/L as CaCO3
- CH 198.00 mg/L as CaCO3
- NCH 293.37 198 95.37 mg/L as CaCO3
- Based on Figure 3-16 the lime dose can be
determined as
Total 277.51
64
Example 3-13 Solution
- Calculate the soda ash dose.
- Since the NCH is equal 95.37 mg/L as CaCO3, and
the magnesium remaining is 40 mg/L as CaCO3, the
amount of NCH removed is - NCH 95.37 40 55.37 mg/L as CaCO3
- The amount of soda ash required to remove the NCH
is - Soda Ash 55.37 mg/L as CaCO3.
65
Example 3-13 Solution
- Calculate the quantity of CaCO3 solids produced
for a design flow of 100 ML/d. - From the bar chart and softening equations (mg/L
as CaCO3) - CO2 44.12 mg/L (Eq 3-61)
- Ca2(CH) (2x198.00) 396 mg/L (Eq. 3-62)
- Ca2(NCH) (238.00 198.00) 40 mg/L (Eq. 3-65)
- Mg2(NCH) (293.53 238.00 - 40) 15.53 mg/L
(Eq. 3-66) - Excess Lime 20 mg/L
- CaCO3 solubility 30 mg/L
66
Softening Example 3-15
- Given the following water analysis, determine the
amount (mg/L) of 90 percent purity CaO and 97
percent purity Na2CO3 that must be purchased to
treat the water to a final hardness of 85 mg/L. - Water Analysis (mg/L as CaCO3)
- CO2 21
- HCO3- 209
- Ca2 183
- Mg2 97
67
Softening Example 3-15
- Solution. First find the TH, CH, and NCH.
- TH Ca2 Mg2 183 97 280 mg/L
- CH HCO3- 209 mg/L
- NCH TH CH 71 mg/L
- Following Figure 3-16, the lime dose as CaCO3
assuming 40 mg/L Mg2 as CaCO3 will be left is
68
Softening Example 3-15
- Solution. Since one mole of CaO equals one mole
of Ca(OH)2, we find 327 mg/L of CaO as CaCO3.
Since the molecular weight of CaO is 56
(equivalent weight 28), and it is 90 purity,
the concentration of CaO is calculated as
69
Softening Example 3-15
- Solution Soda Ash Requirement. The amount of NCH
that can be left in solution is equal to the
final hardness desired (85 mg/L) minus the CH
left behind due to solubility, etc.. (40 mg/L)
and is equal to 85 40 45 mg/L. - The NCH reacted is equal to the initial NCH (71
mg/L) minus the NCH which can be left (45 mg/L)
and is 71 45 26 mg/L, so from Figure 3-16,
Na2CO3 26 mg/L as CaCO3
70
Types of Lime Softening Configurations
- Single stage softening
- Calcium removal only
- Flash mixer followed by reactor/clarifier
- For non carbonate hardness, soda ash is added
before or after the flash mixer - The ph about 10.2 to 10.5 after the flash
mixer. - Waste sludge CaCO3 is recycled to the head of
the process train to improve the efficiency
softening process. - The second stage of CO2 recarbonation is used to
lower the pH value around 8.3 to 8.5 - Filtration is used to removal any particles
formed after recarbonation as CaCO3.
71
Solids Contactor - Flocculator Clarifier
72
Solids Contactor - Flocculator Clarifier
73
Types of Lime Softening Configurations
- Single stage softening
- Calcium removal only
- Flash mixer followed by reactor/clarifier
- For non carbonate hardness, soda ash is added
before or after the flash mixer - The ph about 10.2 to 10.5 after the flash
mixer. - Waste sludge CaCO3 is recycled to the head of
the process train to improve the efficiency
softening process. - The second stage of CO2 recarbonation is used to
lower the pH value around 8.3 to 8.5 - Filtration is used to removal any particles
formed after recarbonation as CaCO3.
74
Solids Contactor - Flocculator Clarifier
75
Solids Contactor - Flocculator Clarifier
76
Types of Lime Softening Configurations
- Two stage softening
- Used when magnesium removal is required from
water with relatively high noncarbonate hardness
- Excess lime is added with a flash mixer to
raise the pH to 11.0 or higher to precipitate
magnesium - CO2 recarbonation is used to reduce the pH to
about 10.0 to 10.6 - Soda ash is added to precipitate the excess
lime added for magnesium removal - The second stage precipitation step is followed
by sedimentation - CO2 is used to bring the pH to 8.3 to 8.5.
- Filtration is used to removal any particles
formed from the second recarbonation step
77
Types of Lime Softening Configurations
- Split stream softening
- Part of the water is treated with excess lime
softening for both calcium and magnesium
hardness removal. In this part, the magnesium
hardness can be removed down to its practical
solubility limit of 10 mg/L - Excess lime is added with a flash mixer to
raise the pH to 11.0 or higher to precipitate
magnesium - The other part of the water is bypassed and
blended with the softened water prior to
sedimentation - The alkalinity of the bypassed raw water is used
to neutralize the excess caustic alkalinity
required to reduce the magnesium in the treated
water - Split treatment works best for groundwaters