Announcements, Feb' 12 - PowerPoint PPT Presentation
1 / 21
Title:
Announcements, Feb' 12
Description:
Reading for today: 172-186 on membrane proteins. ... connexin: 4 positive peaks from hydropathy analysis predicts the protein has 4 ... – PowerPoint PPT presentation
Number of Views:55
Avg rating:3.0/5.0
Title: Announcements, Feb' 12
1
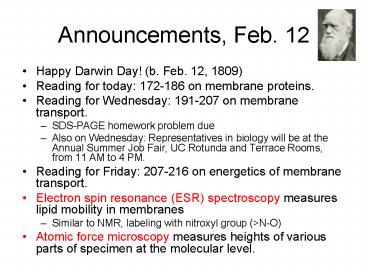
Announcements, Feb. 12
- Happy Darwin Day! (b. Feb. 12, 1809)
- Reading for today 172-186 on membrane proteins.
- Reading for Wednesday 191-207 on membrane
transport. - SDS-PAGE homework problem due
- Also on Wednesday Representatives in biology
will be at the Annual Summer Job Fair, UC Rotunda
and Terrace Rooms, from 11 AM to 4 PM. - Reading for Friday 207-216 on energetics of
membrane transport. - Electron spin resonance (ESR) spectroscopy
measures lipid mobility in membranes - Similar to NMR, labeling with nitroxyl group
(gtN-O) - Atomic force microscopy measures heights of
various parts of specimen at the molecular level.
2
Suggestions for studying from your fellow
students (gt90 on Exam 1)
- I went to the study session, had a study group,
and read over the powerpoints. - I read through the powerpoint slides.
- I started studying 5 days before studied 2
sets of notes each night then all on the
Thursday night before the test. I also looked at
diagrams on the power points. - I rewrote my notes and made notes from that on
the stuff I didnt know as well. Then studied
mainly that material. - Re-wrote notes, made up questions and
answered.
3
Outline/Learning Objectives
- Membrane Proteins
- SDS-PAGE
- Membrane proteins in red blood cells
- Classes of MB proteins
- Orientation and glycosylation of MB proteins
- After reading the text, attending lecture, and
reviewing lecture notes, you should be able to - Explain how proteins can be separated by
SDS-PAGE, and apply your knowledge to solve
SDS-PAGE problems. - Describe the classes of membrane proteins and how
they can be removed from membranes.
4
A. SDS-PAGE
5
SDS-PAGE Reagents
- Sodium dodecyl sulfate
- Strong ionic detergent
- Removes proteins from MB, solubilizes hydrophobic
amino acids - Unfolds and coats proteins w/ negative charge
- Triton X-100
- Mild non-ionic detergent
- Removes proteins from MB
- Solubilizes proteins but does not unfold them.
6
SDS-PAGE Reagents
- Polyacrylamide forms gel matrix
- small proteins go through fast
- large proteins go through slowly
- ?-mercaptoethanol breaks S-S bonds
- with reducing conditions break subunits apart
- without non-reducing conditions keep subunits
together - Allows determination of number of subunits
HS-CH2-CH2-OH
7
B. RBC membrane proteins
8
Functions of MB Proteins
- Receptors or signals, e.g. glycophorin
- Structural, e.g. spectrin, ankyrin, Band 4.1
- Transporters, e.g. glucose transporter, Band 3
- Channels, e.g. Na channels in excitable cells
- Enzymes, e.g. G3PDH
- Electron transport proteins in mitochondria,
chloroplasts - Intercellular adhesion and communication, e.g.
gap junctions
9
Evidence for mosaic of proteins Freeze-fracture
SEM
10
Freeze-Fracture SEM of membranes
lt Artificial bilayer w/o protein Artificial
bilyaer w/protein gt
11
C. Classes of Membrane Proteins
transmembrane
1. Integral membrane proteins require detergent
to remove from MB 2. Peripheral membrane
proteins removed by milder treatments 3.
Lipid-anchored membrane proteins in lipid rafts
12
Solubilization of integral membrane protein by
nonionic detergent
Critical micelle concentration
13
SDS-PAGE Problem
- You are given a preparation of kangaroo membranes
(M), part of which looks like this (assume
membrane is sealed) - You do the following experiment, where an arrow
indicates centrifugation - You also treat M with protease, on the side of
the bilayer indicated in the diagram. This
sample is called PRO. - All samples (M, S1, P1, S2, P2, PRO) are mixed
with SDS and run on a denaturing polyacrylamide
gel. Diagram what you expect to see in the gel
for each sample.
Protease
Protein B
Out In
membrane
Protein A
Isolated membranes (M)
Sup S1 Pel P1
Add non-ionic detergent (to solubilize membranes)
Sup S2 Pel P2
Salt wash
Spin
Spin
14
Solution and Homework
- Part 1
- Part 2 You then get adventurous, and look at
the membrane from an aardvark cell, repeating the
exact same protocols as for the kangaroo
membranes. You also run a gel, as above, and see
this - Homework Draw a diagram of what the aardvark
membrane looks like (Hint the protein may cross
the membrane more than once).
15
1. Integral Membrane Proteins
- Strong treatments (detergents) are required to
remove from MB. - Amphipathic molecules
- Transmembrane regions are ? -helical with
hydrophobic R groups facing out - usually 20-30
amino acids.
16
2. Peripheral and 3. Lipid-Anchored Membrane
proteins
- Peripheral MB proteins
- Weak treatments (change in pH or ionic strength,
removal of Ca2) remove from MB since bound by
electrostatic interactions or H-bonds. - Can be on outside or inside spectrin, ankyrin,
and Band 4.1 are inside examples from RBCs.
- Lipid-anchored MB proteins
- Covalently bound to membrane lipids.
- Most bound to fatty acids on inner leaflet.
- Some bound to outer leaflet linked to GPI (a
glycolipid in external monolayer) - May be enriched in lipid rafts
17
Glycophorin and Bacteriorhodopsin
- Bacteriorhodopsin was one of first membrane
proteins whose 3D structure was determined. - It functions as a light-driven proton pump
18
D. Many membrane proteins are glycosylated
- In addition to lipids and proteins, most
membranes have significant amounts of
carbohydrates - Erythrocyte - 52 protein, 40 lipid, 8 carb.
- Glycolipids account for only small portion of
membrane carbohydrates most is in form of
glycoproteins. - Addition of carbohydrate side chain to a protein
is glycosylation.
19
N-linked and O-linked glycosylation
- Linkage to either N or O on R groups
- Function of glycoproteins usually in plasma
membranes, role in cell-cell recognition along
with membrane receptors - Glycosylation occurs in ER and Golgi
20
Chains vary from 2-60 sugar units
21
Purpose of protein glycosylation
- Synthesis of complex carbohydrates requires a
separate enzyme for each different step, unlike
other polymerization reactions. - May be several functions, not well-understood
- Presence of oligosaccharides makes glycoprotein
more resistant to digestion by extracellular
proteases. - Glycosylation also may be important for
receptor-ligand binding. - CHO-binding proteins are called lectins







![Funding and acquisitions in Indian startups this week [12 – 17 Feb] PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10001240.th0.jpg?_=20240220015)






















