Molecular Orbitals - PowerPoint PPT Presentation
1 / 16
Title:
Molecular Orbitals
Description:
We used the hydrogenic orbitals as the energy states for electrons in all atoms. ... thing now: we are going to construct molecular orbitals out of hydrogenic atomic ... – PowerPoint PPT presentation
Number of Views:90
Avg rating:3.0/5.0
Title: Molecular Orbitals
1
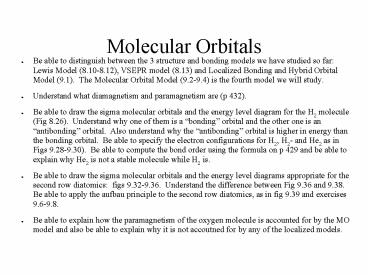
Molecular Orbitals
- Be able to distinguish between the 3 structure
and bonding models we have studied so far Lewis
Model (8.10-8.12), VSEPR model (8.13) and
Localized Bonding and Hybrid Orbital Model (9.1).
The Molecular Orbital Model (9.2-9.4) is the
fourth model we will study. - Understand what diamagnetism and paramagnetism
are (p 432). - Be able to draw the sigma molecular orbitals and
the energy level diagram for the H2 molecule (Fig
8.26). Understand why one of them is a bonding
orbital and the other one is an antibonding
orbital. Also understand why the antibonding
orbital is higher in energy than the bonding
orbital. Be able to specify the electron
configurations for H2, H2- and He2 as in Figs
9.28-9.30). Be able to compute the bond order
using the formula on p 429 and be able to explain
why He2 is not a stable molecule while H2 is. - Be able to draw the sigma molecular orbitals and
the energy level diagrams appropriate for the
second row diatomics figs 9.32-9.36.
Understand the difference between Fig 9.36 and
9.38. Be able to apply the aufbau principle to
the second row diatomics, as in fig 9.39 and
exercises 9.6-9.8. - Be able to explain how the paramagnetism of the
oxygen molecule is accounted for by the MO model
and also be able to explain why it is not
accoutned for by any of the localized models.
2
Models (so far)
- Lewis Model(8.10-8.12)
- VSEPR Model (8.13)
- Hybrid Orbital and Localized Electron Model
(Section 9.1) - Molecular Orbital Model (9.2-9.3)
3
Why Do We Need Another Model?
- 1) The first 3 models are localized bonding
models. They view chemical bonding as a sharing
of electrons between two atoms. Even the hybrid
orbital model is constructing atomic orbitals
about a central atom. - Clearly there are problems with localization
one big one is the dicey issue of resonance
structures which show that electrons can be
shared by more than two atoms and can be spread
out over a molecule this sharing of electrons
among several bonds is called delocalization. - 2) Another problem is the magnetism
(paramagnetism) of molecular oxygen (Fig 9.40).
Magnetism means that molecular oxygen has
unpaired electron spins. None of the other 3
models can account for this. - 3) The MO Model attempts to fix these two
problems and come up with a more comprehensive
theory.
4
Problem is...
- The MO model is most satisfactory only for
homonuclear diatomic molecules. - You can generalize it, but that's beyond the
scope of this course. - Nevertheless, we will look at it now just to see
what it is like.
5
Overview of the MO Model
- Remember how we treated atoms? We used the
hydrogenic orbitals as the energy states for
electrons in all atoms. We then built up
electron configurations by adding electrons to
those energy levels using the aufbau principle
we added the electrons to the orbitals following
the Pauli Principle, Hund's Rule and the orbital
filling order. - We are going to do a totally analogous thing now
we are going to construct molecular orbitals out
of hydrogenic atomic orbitals, and then build up
electron configurations by adding electrons to
those energy levels using the aufbau principle
we will add electrons to the orbitals following
the Paului Principle, Hund's Rule and the orbital
filling order.
6
The Hydrogen (H2) Molecule
- Here are the two molecular orbitals you can make
out of the hydrogen 1s orbital one is called a
bonding sigma orbital and the other is called an
antibonding sigma orbital. - Notice that (unlike hybrid orbitals, where we are
combining orbitals on one single atom) here we
are combining orbitals on two different atoms. - The antibonding orbital has a node between the
two nuclei.
7
We Also Get an Energy Level Diagram...
- We know that the energy of the system is lowered
by the bond. - Energy conservation says that there must also be
a state that is higher by the same amount of
energy. We suppose that it is the sigma orbital
that is higher in energy.
8
Let's Put It All Together Now...(Fig 9.26)
9
AufBau Principle
- We can now describe H2, H2- and He2 using the
aufbau principle. - Here is H2
10
Here is H2- (left, fig 9.29) and He2 (right, fig
9.30)
11
Bond Order (BO)
- New definition of bond order (p 429)
- Bond order (½) (number of bonding electrons
number antibonding electrons) - e.g. H2 BO 1 He2 BO0
12
We Can Construct a Similar Model for All
Homonuclear Diatomic Molecules for Second Row
Elements(Fig 9.32)
13
The P Orbitals We'll Use (Fig 9.33)
14
The MO's We'll Make from the P Orbitals (Fig 9.34
and 9.35)
15
AufBau Principle for Second Row Diatomics (Fig
9.39)
16
Summary of MO Theory
- The BO's match well with the Lewis Model
- Oxygen is paramagnetic!!!
- Delocalization makes a lot more sense.