Phase Transformation Basic Concepts - PowerPoint PPT Presentation
1 / 12
Title:
Phase Transformation Basic Concepts
Description:
436-220 Unit 2: Engineering Materials Dr. K. Xia 1. Phase Transformation - Basic Concepts ... H: enthalpy - heat content of the system ... – PowerPoint PPT presentation
Number of Views:807
Avg rating:3.0/5.0
Title: Phase Transformation Basic Concepts
1
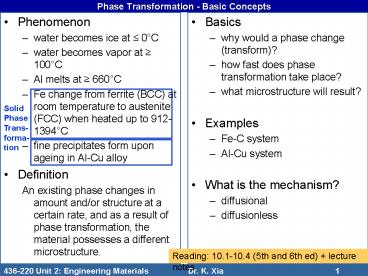
Phase Transformation - Basic Concepts
- Phenomenon
- water becomes ice at 0C
- water becomes vapor at 100C
- Al melts at 660C
- Fe change from ferrite (BCC) at room temperature
to austenite (FCC) when heated up to 912-1394C - fine precipitates form upon ageing in Al-Cu alloy
- Definition
- An existing phase changes in amount and/or
structure at a certain rate, and as a result of
phase transformation, the material possesses a
different microstructure.
- Basics
- why would a phase change (transform)?
- how fast does phase transformation take place?
- what microstructure will result?
- Examples
- Fe-C system
- Al-Cu system
- What is the mechanism?
- diffusional
- diffusionless
Solid Phase Trans-forma-tion
Reading 10.1-10.4 (5th and 6th ed) lecture
notes
2
Phase Transformation - Basic Concepts
- Thermodynamics
- Gibbs free energy (G)
- a measurement of the state of a system (e.g. an
alloy) - the work required to change a system from one
state to another state - consisting of two parts
- G H TS
- H enthalpy - heat content of the system
- S entropy - randomness (degree of chaos) of
the system - T absolute temperature
- H E PV
- E internal energy of the system (kinetic
potential energies) - P pressure
- V volume
- (for solid and liquid, E PV. Thus H E)
- G is used to determine the stability of a system
- a system with a higher G would tend to change
into a system with a lower G.
Additional reading - 1.1 in Phase
Transformations in Metals and Alloys by D. A.
Porter K. E. Easterling, 1992. - Any textbook
on thermodynamics.
3
Phase Transformation - Basic Concepts
- Phase equilibrium
- a system is in equilibrium if it has no desire to
change, i.e. it is in a state of minimum G - mathematically, this means
- dG 0 (and d2G gt 0)
- Stable and metastable state
- structure A is absolutely stable
- structure B is only relatively stable -
metastable - structure B has the tendency to become A to
possess lower G
G
dG 0
dG 0
Metastable state
Stable state
A
B
"Structure" of the system
4
Phase Transformation - Basic Concepts
- Phase transformation
- Driving force
- A phase (S) will change into a new phase (F) if
the resulting system (phase) has a lower G - DG G2 G1 lt 0
- DG is the driving energy
- Barrier
- However, there is often an transitional,
activated state (A) between S and F. - In order for transformation S ? F to take place,
an activation energy, DGa, has to be provided
(usually by thermal fluctuation), e.g. if the
transformation is through diffusion.
3
2
1
2
E
1
3
G'
DGa activation energy
G
G1
DG driving energy
G2
F
A
S
(Initial State)
(Activated State)
(Final State)
5
Phase Transformation - Basic Concepts
- Kinetics
- how fast is a phase transformation taking place?
- the transformation rate is proportional to
- exp ( DGa/RT)
- the higher the activation energy, the slower the
transformation - temperature very important (again refer to Chap.
5 Diffusion) - example
- diamond is metastable at RT, but will not
transform to become graphite (the stable phase)
due to very high activation energy
- precipitation process takes place at various
rates depending on temperature - Thermodynamics determines whether a phase
transformation is likely to take place (whether
there is a driving force). - But, it is the activation energy and temperature
which determine whether a transformation can
actually happen and at what rate. It is often
the kinetics that determines which microstructure
will exist, not thermodynamics.
6
Phase Transformation - Basic Concepts
- Process and kinetics of phase transformation
- How does a phase transformation start and
progress? - Example solidification of a pure material (such
as water and liquid aluminium) - a transformation
from liquid phase to solid phase - formation of small solid nuclei fromliquid phase
nucleation - each solid crystal becomes larger growth
- continuing growth of these crystals (called
grains) until all liquid is consumed
Reading 10.3W of the 6th ed downloadable from
"www.wiley.com/college/callister" or from CD
available at the reserve desk of Engineering
Library
7
Phase Transformation - Basic Concepts
- Homogeneous nucleation
- a sphere of radius r of a solid forms in a liquid
- GL free energy per volume of liquid
- GS free energy per volume of solid
- g surface free energy per area of the
solid-liquid interface
- free energy change per volume
- DGV GS GL
- (GS lt GL below the melting point, i.e. DGV is
negative) - total free energy change
driving force
barrier force
8
Surface Energy
Atoms with higher energy
The yellow atoms are on the surface and have less
bonds compared to those in the interior (the
green ones). This is because bonding reduces the
potential energy of the atoms. The surface,
therefore, has higher energy.
9
Phase Transformation - Basic Concepts
- It is seen that the total free energy change (DG)
increases with r at r lt r and decreases with r
at r gt r. - An initial crystal of gt r will grow since an
increase in size results in a decrease in G.
This crystal thus is a real nucleus.
- Similarly, an initial crystal of lt r will shrink
and disappear to reduce G. It is thus not a real
nucleus, only an embryo. - r is called the critical radius
- In order to form a real nucleus, a critical free
energy, DG, has to be provided. This DG is the
so-called activation free energy which is usually
provided through thermal fluctuation. - r and DG can be obtained by letting d(DG)/dr 0
G
r
DG
10
Phase Transformation - Basic Concepts
- the number of stable nuclei (those crystals
having radii gt r) is - It is clear that the larger the DG the more
difficult the nucleation is. - DG also depends on T although it is implicit
here. In fact, temperature affects DG more
significantly. - In addition, the phase transformation process
often involves the diffusion of atoms which has
its own activation energy, Qd.
- The total nucleation rate (nuclei per volume per
second)
k Boltzmann's constant T absolute temperature
nucleation rate is the highest at an intermediate
temperature
diffusion easier less driving force
diffusion more difficult more driving force
DG decreases with DT
Self study Heterogeneous Nucleation
Rate
11
Phase Transformation - Basic Concepts
- Growth
- through diffusion
- growth rate
- Q activation energy (often Q Qd)
- Fraction of transformation, y
- how much of the parent phase has been transformed
- for solid state transformation at a certain
temperature
Temperature is often the most important and used
to induce and control a phase transformation
Avrami equation
Overall transformation rate is the highest at an
intermediate temperature
time for the transformation to proceed to halfway
to completion
12
Phase Transformation - Basic Concepts
- Summary
- Phases in a given system may change with external
conditions (most importantly, with temperature). - Thermodynamics determines whether a phase is
stable or a transformation is possible (the Gibbs
free energy is one measurement used for this
purpose). - The activation energy, arising from barriers to
transformation, determines whether and how fast a
transformation would actually take place.
- A transformation starts by nucleation of the new
phase in which a critical size exists and a
critical (activation) energy is needed. - The nuclei will grow, often through diffusion,
until the old phase is replaced. The growth rate
is the highest at some intermediate temperature. - The whole phase transformation takes some time to
complete (this can be very long indeed!).