Australia Medical Devices PowerPoint PPT Presentations
All Time
Recommended
Reprocessed Medical Devices Market 2018-2024: This research reviews the overall reprocessed medical devices industry, which according to a report by Global Market Insights, Inc., is growing at more than 14% CAGR during 2017 and 2024 to hit the $3bn revenue mark.
| PowerPoint PPT presentation | free to download
Joya Medical Supplies is your top destination for quality medical supplies in Sydney, Australia. From PPE to equipment and healthcare products, find all your medical needs. Explore our collection right now!
| PowerPoint PPT presentation | free to download
Australia has implemented a new regulatory framework for CE mark for medical devices, moving away from the CE marking system used in Europe. The Therapeutic Goods Administration (TGA) in Australia is responsible for regulating medical devices. It's essential to note that regulations and requirements can change over time. I recommend checking the latest information on the TGA website or consulting with our regulatory expert to ensure compliance with current regulations regarding medical devices in Australia
| PowerPoint PPT presentation | free to download
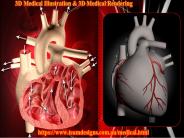
Team designs create realistic 3D medical illustration images difficult to photograph for the medical field. 3D medical rendering include internal organs, eternal organs, microscopic objects, pathological processes, complex medical concepts and pharmaceutical products. These high quality 3D images are used for advertising in medical presentations, marketing, fairs, multimedia and video using 3D modeling and 3D rendering. High impact 3D images help to market and promote new advanced medical devices. For more info, Visit our website here: https://www.teamdesigns.com.au/medical.html
| PowerPoint PPT presentation | free to download
Bharat Book Bureau Provides the Trending Market Research Report; on “Vision Care Devices - Medical Devices Pipeline Assessment, 2017”, (http://www.bharatbook.com/medical-devices-market-research-reports-749040/vision-care-devices.html)The report provides comprehensive data on the pipeline merchandise with comparative analysis of the merchandise at numerous stages of development, reviews major players & data regarding clinical trials current.
| PowerPoint PPT presentation | free to download
Avail more information from Sample Brochure of report @ https://goo.gl/2ODCDl A detailed qualitative analysis of the factors responsible for driving and restraining growth of the Global Wearable Medical Devices Industry Market and future opportunities are provided in the report.
| PowerPoint PPT presentation | free to download
Get Detailed TOC and Overview of Report @https://www.htfmarketintelligence.com/report/global-medical-devices-materials-market
| PowerPoint PPT presentation | free to download
Get Detailed TOC and Overview of Report @ https://www.htfmarketintelligence.com/report/global-medical-suction-devices-market
| PowerPoint PPT presentation | free to download
In Australia, medical devices are regulated by the Therapeutic Goods Administration (TGA). To gain approval for a medical device technical file in Australia, manufacturers are required to create a Technical File or Technical Documentation, which contains detailed information about the design, manufacturing, and performance of the device. Consulting with regulatory experts or the TGA is advisable to ensure that your Technical File meets all necessary criteria.
| PowerPoint PPT presentation | free to download
Australia has implemented a new regulatory framework for CE mark for medical devices, moving away from the CE marking system used in Europe. The Therapeutic Goods Administration (TGA) in Australia is responsible for regulating medical devices. It's essential to note that regulations and requirements can change over time. I recommend checking the latest information on the TGA website or consulting with our regulatory expert to ensure compliance with current regulations regarding medical devices in Australia
| PowerPoint PPT presentation | free to download
Wearable medical devices are small electronic products, often consisting of one or more sensors, and having computational capability. They are embedded into items that are attach to the body parts, such as head, feet, arms, wrists and waist. They can resemble a watch, eyeglasses, some clothing, contact lenses, shoes or even jewellery.
| PowerPoint PPT presentation | free to download
Implantable Medical Devices NSF Project * * * * * * * * * Electromagnetic compatibility refers to a kind of environmental equilibrium. In this case, the environment ...
| PowerPoint PPT presentation | free to download
The medical devices market is expected to reach US$ 767,684.9 million by 2027 from US$ 483,285.8 million in 2019. The market is estimated to grow with a CAGR of 6.1% from 2020 to 2027. https://www.theinsightpartners.com/reports/medical-devices-market
| PowerPoint PPT presentation | free to download
JK MEDIRISE is a healthcare startups company in the international medical devices market. JK MEDIRISE is decade old believing in the statement to deliver the premier quality and international standard Medical device products for the healthcare and medical sector. # Products: * DENTAL IMPLANTS * TRANSFUSION / INFUSION * INTERVENTIONAL CARDIOLOGY * UROLOGY * GASTROENTEROLOGY * ANAESTHESIA * SURGERY * SURGICAL DRESSINGS * SURGICAL DISPOSABLE PRODUCT * MISCELLANEOUS JK MEDIRISE would like to invite you to consider possible business collaborations. Please visit our company website for more information. I am looking forward to hearing from you. With all respect and best regards, Mr. Ketan MUNJANI JK Medirise
| PowerPoint PPT presentation | free to download
The active implantable medical devices market accounted to US$ 39,026.9 Mn in 2017 and is expected to grow at a CAGR of 7.9% during the forecast period 2018 - 2025, to account to US$ 22,096.9 Mn by 2025. https://www.theinsightpartners.com/reports/active-implantable-medical-devices-market
| PowerPoint PPT presentation | free to download
Reprocessing is a regulated activity for manufacturing the medical devices that are conducted by third party or hospitals. To read more: https://www.goldsteinresearch.com/report/global-reprocessed-medical-devices-market
| PowerPoint PPT presentation | free to download
Looking for a health care job in Australia? Here is your number one portal in Australia for healthcare jobs, Review and apply now!!
| PowerPoint PPT presentation | free to download
Wearable medical devices are electronic and have sensors that help in monitoring as well as keeping track of a patient's health. Some of the uses of these devices include activity tracking, infant monitoring, and vital signs monitoring, among others.
| PowerPoint PPT presentation | free to download
According to our new market research study on “Medical Devices Market to 2027 – Global Analysis and Forecast – by Product Type, Function, and End User,” the market is expected to reach US$ 767,684.9 million by 2027 from US$ 483,285.8 million in 2019; it is estimated to grow at a CAGR of 6.1% from 2020 to 2027. The report highlights trends prevailing in the global medical devices Market and the drivers and restraints pertaining to the market growth.
| PowerPoint PPT presentation | free to download
Wearable Medical Devices Market is projected to reach US$ 23,310.9 million by 2025; it is expected to register a CAGR of 18.1% from 2018 to 2025
| PowerPoint PPT presentation | free to download
The global wearable medical devices market is forecast to grow at a CAGR (compound annual growth rate) of around 20.9 % to 2022.
| PowerPoint PPT presentation | free to download
The nitinol medical devices market was valued at US$ 15,807.53 million in 2020 and is projected to reach US$ 27,327.25 million by 2028; it is expected to grow at a CAGR of 7.2% during 2021-2028.
| PowerPoint PPT presentation | free to download
The active implantable medical devices market accounted to US$ 39,026.9 Mn in 2017 and is expected to grow at a CAGR of 7.9% during the forecast period 2018 - 2025, to account to US$ 22,096.9 Mn by 2025.
| PowerPoint PPT presentation | free to download
The 3D printing medical devices market is projected to reach US$ 6,583.50 million by 2028 from US$ 2,123.11 million in 2021; it is estimated to grow at a CAGR of 17.5% from 2021 to 2028.
| PowerPoint PPT presentation | free to download
According to the latest research report by IMARC Group, The global wearable medical devices market size reached US$ 23.1 Billion in 2022. Looking forward, IMARC Group expects the market to reach US$ 73 Billion by 2028, exhibiting a growth rate (CAGR) of 22% during 2023-2028. More Info:- https://www.imarcgroup.com/wearable-medical-devices-market
| PowerPoint PPT presentation | free to download
The reprocessed medical devices market is projected to reach US$ 5,653.87 million by 2028 from US$ 2,087.36 million in 2021; it is expected to grow at a CAGR of 15.3% from 2021 to 2028.
| PowerPoint PPT presentation | free to download
The Therapeutic Goods (Medical Devices) Act & Regulatory Compliance for Assistive Care Equipment Manufacturers and Suppliers. Acknowledgements to
| PowerPoint PPT presentation | free to view
The pediatric medical devices market is expected projected to reach US$ 48,305.21 million in by 2028 from an estimated value of US$ 28,207.65 million in 2021. The market it is estimated to grow with at a CAGR of 8.0% from during 2021–2028.
| PowerPoint PPT presentation | free to download
According to our latest study on “Home Medical Devices Market Forecast to 2028 – COVID-19 Impact and Global Analysis – by Functionality, Services, Distribution Channel, and Geography,” the market is expected to reach US$ 57,102.9 million in 2028 from US$ 33,635.2 million in 2020; it is expected to grow at a CAGR of 7.0% from 2021 to 2028. The home medical devices market growth is attributed to the growing demand for homecare medical devices by the geriatric population, increasing homecare settings, and rising market consolidation. However, stringent regulations, challenges, and risks for homecare medical devices are expected to hamper the growth of the home medical devices market. The home medical devices market report also studies the ongoing opportunities and home medical devices market trends that can have a significant impact on the market growth potential.
| PowerPoint PPT presentation | free to download
The ‘Contract Regulatory Affairs Management Market for Medical Devices, 2019-2030’ report features a detailed study on the current landscape of contract service providers focused on regulatory affairs management for medical devices.
| PowerPoint PPT presentation | free to download
Looking forward, the wearable medical devices market value is projected to reach a strong growth during the forecast period (2021-2026). More info:- https://www.imarcgroup.com/wearable-medical-devices-market
| PowerPoint PPT presentation | free to download
Reprocessed Medical Devices Market: Cardiology Devices Segment Slated to Dominate the Regional Market Through 2027: Asia Pacific Industry Analysis and Opportunity Assessment, 2017-2027
| PowerPoint PPT presentation | free to download
Bharat Book Bureau Provides the Trending Market Research Report; on “Spinal Fusion Systems - Medical Devices Pipeline Assessment, 2017”, (https://www.bharatbook.com/medical-devices-market-research-reports-803006/spinal-fusion-systems.html)The report provides comprehensive info on the pipeline merchandise with comparative analysis of the merchandise at varied stages of development and reviews major players concerned within the pipeline development.
| PowerPoint PPT presentation | free to download
Anti-microbial coatings segment is the largest product segment, accounting for more than one-third share of the global market. Hydrophilic coatings segment is projected tbe the fastest growing product segment during the analysis period. However, Cardiovascular is expected tbe the fastest growing end-user segment. North America accounts for the majority share in medical device coating technology market, followed by Europe. However, Asia Pacific is expected twitness highest CAGR in forecast period due trising medical products manufacturing in the region.
| PowerPoint PPT presentation | free to download
Goldstein Research analyst forecast the Australia medical device market size is set to reach USD 4.56 billion by 2025, at a CAGR of 10% over the forecast years.
| PowerPoint PPT presentation | free to download
Australia Cardiovascular Devices Market Outlook to 2020, provides key market data on the Australia Cardiovascular Devices market. The report provides value, in millions of US dollars, and volume (in units) and average price data (in US dollars), within market categories – Cardiac Assist Devices, Cardiac Rhythm Management Devices, Cardiovascular Monitoring and Diagnostic Devices, Cardiovascular Prosthetic Devices, Cardiovascular Surgery Devices, Electrophysiology Devices, External Defibrillators, Interventional Cardiology, Peripheral Vascular Devices and Prosthetic Heart Valves. See Full Report @ bit.ly/1zZlT3m
| PowerPoint PPT presentation | free to download
Get a detailed report at http://www.marketoptimizer.org/australia-cardiac-assist-devices-market-outlook-to-2020.html . The data in the report is derived from dynamic market forecast models. Researcher uses epidemiology and capital equipment-based models to estimate and forecast the market size. The objective is to provide information that represents the most up-to-date data of the industry possible. The epidemiology-based forecasting model makes use of epidemiology data gathered from research publications and primary interviews with physicians to establish the target patient population and treatment flow patterns for individual diseases and therapies. Using prevalence and incidence data and diagnosed and treated population, the epidemiology-based forecasting model arrives at the final numbers.
| PowerPoint PPT presentation | free to download
Get a detailed report at http://www.marketoptimizer.org/australia-prosthetic-heart-valves-market-outlook-to-2020.html . Researcher’s new report, “Australia Prosthetic Heart Valves Market Outlook to 2020″, provides key market data on the Australia Prosthetic Heart Valves market. The report provides value, in millions of US dollars, and volume (in units) and average price data (in US dollars), within market categories Mechanical Heart Valves, Tissue Heart Valves and Transcatheter Heart Valves. The report also provides company shares and distribution shares data for the market category, and global corporate-level profiles of the key market participants, pipeline products, and news and deals related to the Prosthetic Heart Valves market wherever available.
| PowerPoint PPT presentation | free to download
The reprocessed medical devices market size is set to reach USD 5.12 billion by 2024, at a CAGR of 15% over the forecast years
| PowerPoint PPT presentation | free to download
Veterinary Medical Equipment Global Market Report 2018 Covering: Carestream Health, IDEXX Laboratories Inc., Agfa Healthcare, MinXray Inc., Diagnostic Imaging Systems provides a comprehensive analysis of global veterinary medical equipment market, latest technologies, and leading vendors in global veterinary medical equipment. To know more, visit https://www.kenresearch.com/agriculture-and-animal-care/animal-care/veterinary-medical-equipment-global-market-report/143760-104.html
| PowerPoint PPT presentation | free to download
Want to buy molnlycke products in Australia? Joya Medical Supplies is one of the trusted medical supplies stores for buying molnlycke products such as dressings, bandage and fixation tape, heel boots, and many more products at low prices. Browse our store and buy online today!
According to the study “Australia Infusion Systems Market Outlook to 2023”, infusion pumps are medical devices used in healthcare facilities to inject fluids such as drugs, nutrients, and blood in a controlled systematic manner. Infusion systems are identified as the fundamental devices in the healthcare and life sciences industry.
| PowerPoint PPT presentation | free to download
United States EEG, EMG and Evoked Potential Devices market competition by top manufacturers/players, with EEG, EMG and Evoked Potential Devices sales volume, price, revenue Million USD and market share for each manufacturer/player; the top players including Cadwell Laboratories, Inc. US Compumedics Limited Australia EB Neuro S.P.A. Italy Electrical Geodesics, Inc. US Lifelines Neurodiagnostic Systems, Inc. US Natus Medical Incorporated US
| PowerPoint PPT presentation | free to download
Big Market Research “Global Medical Device Market” Size, Share, Industry Trends, Demand, Insights, Analysis, Research, Report, Opportunities, Company Profiles, Forecast to 2018. Visit for more info @ http://www.bigmarketresearch.com/global-medical-device-to-2018-size-growth-and-forecasts-in-nearly-70-countries-market Global Medical Device Market to 2018 - Market Size, Growth, and Forecasts in Nearly 70 Countries is a comprehensive publication that enables readers the critical perspectives to be able to evaluate the world market for medical devices. The publication provides the market size, growth and forecasts at the global level as well as for the following countries: Argentina, Australia, Belgium
| PowerPoint PPT presentation | free to download
Medical device companies who wish to sell their products in their respective countries must adhere to the regulations provided by the country’s regulatory bodies for labeling medical devices.
| PowerPoint PPT presentation | free to download
Paragon Care is a dynamic supplier of healthcare equipment, devices, and consumables. It is a leading company which is also an ASX listed company and has acquired many healthcare sector brands. Now getting medical products in Australia is no more a hassle. Paragon Care will take care of all your requirements.
The Australia laser hair removal market is anticipated to witness substantial growth during the forecast period. The major factors driving the growth of the Australia laser hair removal market is due to the increase in the demand for seeking alternatives to traditional hair removal methods, such as shaving or waxing, due to the convenience and effectiveness of laser treatments. In addition, rise in disposable income and changing beauty standards contribute toward the growth of the market.
| PowerPoint PPT presentation | free to download
For the best emergency medical equipment for your hospital, get in touch with Paragon Care. With the largest range of products in Australia, Paragon Care is the right choice for any medical facility.
| PowerPoint PPT presentation | free to download
MDSAP is a technique through which medical device makers may be inspected once for conformity with the different medical device markets standards and regulatory requirements. Multiple individual audits or inspections by participating regulatory bodies or their representatives are replaced by a single audit. As a result, the Medical Device Single Audit Program minimizes the overall number of audits or inspections and optimises the time and resources spent on audit operations for many medical device manufacturers.
| PowerPoint PPT presentation | free to download
It is important to note that nicotine-free vape supplies, devices, and e-liquids can still be sold and purchased in most of the states and territories in Australia. These products can be found in online retail stores and other tobacco retail outlets. As an Ejuice manufacturer, you should be able to meet the diverse needs of vapers and also meet the government regulations to the evolving health and safety concerns associated with vaping. Visit - https://www.oz-eliquid.com.au/
| PowerPoint PPT presentation | free to download
Medical Equipment Maintenance Market to 2027 - Global Analysis and Forecast by Device Type (Electromedical Equipment, Endoscopic Devices, Surgical Instruments, and Other Medical Equipment); Service Type (Preventive Maintenance, Corrective Maintenance and Operational Maintenance); Service Provider (Original Equipment Manufacturers, Independent Service Organizations, and In-House Maintenance) and Geography
| PowerPoint PPT presentation | free to download
The global medical polymers market size reached US$ 21.9 Billion in 2023. Looking forward, IMARC Group expects the market to reach US$ 39.1 Billion by 2032, exhibiting a growth rate (CAGR) of 6.5% during 2024-2032.
| PowerPoint PPT presentation | free to download
Split by applications, this report focuses on sales, market share and growth rate of Medical Oxygen Systems in each application https://www.bharatbook.com/medical-devices-market-research-reports-813244/global-top-countries-medical-oxygen-systems.html
| PowerPoint PPT presentation | free to download
The Global Vital Signs Monitoring Devices market size will be million in 2022, from the million in 2016, with a (Compound Annual Growth Rate) from 2016 to 2022. https://www.bharatbook.com/medical-devices-market-research-reports-878351/global-top-countries-vital-signs-monitoring-devices.html
| PowerPoint PPT presentation | free to download
The global vascular closure devices market size reached US$ 1.5 Billion in 2023. Looking forward, IMARC Group expects the market to reach US$ 2.4 Billion by 2032, exhibiting a growth rate (CAGR) of 5.1% during 2024-2032.
| PowerPoint PPT presentation | free to download
The global medical injection molding market size reached US$ 23.3 Billion in 2023. Looking forward, IMARC Group expects the market to reach US$ 34.1 Billion by 2032, exhibiting a growth rate (CAGR) of 4.2% during 2024-2032.
| PowerPoint PPT presentation | free to download